Abstract
Background
CD47 has been identified as an innate immune checkpoint and found to be associated with inferior survival in various types of cancer. However, the critical role of CD47 in gastric cancer and its association with tumor associated macrophages remain unclear.
Methods
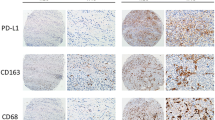
Tumor tissues of gastric cancer from Zhongshan Hospital and data from GSE62254, GSE84437 and TCGA datasets were analyzed. Immunohistochemistry was performed to detect the expression of CD47, CD11c, CD163 and CD68 in gastric cancer tissues. Kaplan–Meier curves and Cox model were used for comparing the clinical outcomes of patients belonging to different subgroups.
Results
Gastric cancer patients with high CD47 expression exhibited poor prognosis and inferior therapeutic responsiveness to fluorouracil-based adjuvant chemotherapy (ACT). A positive correlation was found between M1-polarized macrophage infiltration and CD47 expression in gastric cancer; however, the prognostic value of M1-polarized macrophages was attenuated in CD47-high gastric cancer patients. Moreover, we found that CD47 mRNA level was enriched in microsatellite-instable (MSI) subtype of gastric cancer and associated with ARID1A mutation and FGFR2 signaling pathway activation.
Conclusions
Aberrant CD47 expression represented an independent predictor for adverse survival outcome and ACT resistance in gastric cancer. Targeting CD47 might be a promising strategy for gastric cancer patients.




Similar content being viewed by others
Data availability
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Zhang upon reasonable request.
Code availability
Not applicable.
References
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. Lancet 388(10060):2654–2664
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820
Group G, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y et al (2010) Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 303(17):1729–1737
Petrillo A, Smyth EC (2020) Multimodality treatment for localized gastric cancer: state of the art and new insights. Curr Opin Oncol 32(4):347–355
O’Donnell JS, Teng MWL, Smyth MJ (2019) Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 16(3):151–167
Rowshanravan B, Halliday N, Sansom DM (2018) CTLA-4: a moving target in immunotherapy. Blood 131(1):58–67
Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X (2015) Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med 21(1):24–33
Pitt JM, Vetizou M, Daillere R, Roberti MP, Yamazaki T, Routy B et al (2016) Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44(6):1255–1269
Rothlin CV, Ghosh S (2020) Lifting the innate immune barriers to antitumor immunity. J Immunother Cancer 8(1):e000695
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F et al (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233(9):6425–6440
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14(7):399–416
Demaria O, Cornen S, Daeron M, Morel Y, Medzhitov R, Vivier E (2019) Harnessing innate immunity in cancer therapy. Nature 574(7776):45–56
Zhang H, Wang X, Shen Z, Xu J, Qin J, Sun Y (2015) Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer 18(4):740–750
Chen J, Zheng DX, Yu XJ, Sun HW, Xu YT, Zhang YJ et al (2019) Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology 8(11):e1652540
Veillette A, Chen J (2018) SIRPalpha-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol 39(3):173–184
Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA et al (2015) CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 21(10):1209–1215
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S et al (2010) Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142(5):699–713
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr et al (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138(2):286–299
Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R et al (2009) CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138(2):271–285
Barrera L, Montes-Servin E, Hernandez-Martinez JM, Garcia-Vicente MLA, Montes-Servin E, Herrera-Martinez M et al (2017) CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer 117(3):385–397
Fu W, Li J, Zhang W, Li P (2017) High expression of CD47 predicts adverse prognosis in Chinese patients and suppresses immune response in melanoma. Biomed Pharmacother 93:1190–1196
Pai S, Bamodu OA, Lin YK, Lin CS, Chu PY, Chien MH et al (2019) CD47-SIRPalpha signaling induces epithelial-mesenchymal transition and cancer stemness and links to a poor prognosis in patients with oral squamous cell carcinoma. Cells 8(12):1658
Yoshida K, Tsujimoto H, Matsumura K, Kinoshita M, Takahata R, Matsumoto Y et al (2015) CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med 4(9):1322–1333
Cao Y, Liu H, Li H, Lin C, Li R, Wu S et al (2017) Association of O6-methylguanine-DNA methyltransferase protein expression with postoperative prognosis and adjuvant chemotherapeutic benefits among patients with stage II or III gastric cancer. JAMA Surg 152(11):e173120
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y et al (2015) Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 12(5):453–457
Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS et al (2011) EMT is the dominant program in human colon cancer. BMC Med Genomics. https://doi.org/10.1186/1755-8794-4-9
Cancer Genome Atlas N (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487(7407):330–337
Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS et al (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 21(5):449–456
Elimova EWR, Shiozaki H, Sudo K, Estrella JS, Badgwell BD, Das P et al (2015) Molecular biomarkers in gastric cancer. J Natl Compr Canc Netw 13(4):e19-29
Hänzelmann SCR, Guinney J (2013) GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. https://doi.org/10.1186/1471-2105-14-7
Wang Y, Xu B, Hu WW, Chen LJ, Wu CP, Lu BF et al (2015) High expression of CD11c indicates favorable prognosis in patients with gastric cancer. World J Gastroenterol 21(31):9403–9412
Guo J, Yu W, Su H, Pang X (2017) Genomic landscape of gastric cancer: molecular classification and potential targets. Sci China Life Sci 60(2):126–137
Kim YS, Jeong H, Choi JW, Oh HE, Lee JH (2017) Unique characteristics of ARID1A mutation and protein level in gastric and colorectal cancer: a meta-analysis. Saudi J Gastroenterol 23(5):268–274
Zhou Y, Shi D, Miao J, Wu H, Chen J, Zhou X et al (2017) PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci Rep 7:43627
Schneider S, Kadletz L, Wiebringhaus R, Kenner L, Selzer E, Fureder T et al (2018) PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis - impact on clinical outcome. Histopathology 73(4):573–584
Marisa L, Svrcek M, Collura A, Becht E, Cervera P, Wanherdrick K et al (2018) The balance between cytotoxic T-cell lymphocytes and immune checkpoint expression in the prognosis of colon tumors. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djx136
Matlung HL, Szilagyi K, Barclay NA, van den Berg TK (2017) The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev 276(1):145–164
Wang J, Lin C, Li H, Li R, Wu Y, Liu H et al (2017) Tumor-infiltrating gammadeltaT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. Oncoimmunology 6(11):e1353858
Wang JT, Li H, Zhang H, Chen YF, Cao YF, Li RC et al (2019) Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann Oncol 30(2):266–273
Genin M, Clement F, Fattaccioli A, Raes M, Michiels C (2015) M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15:577
Mizuguchi A, Takai A, Shimizu T, Matsumoto T, Kumagai K, Miyamoto S et al (2018) Genetic features of multicentric/multifocal intramucosal gastric carcinoma. Int J Cancer 143(8):1923–1934
Pectasides E (2016) Genomic alterations and targeted therapy in gastric and esophageal adenocarcinoma. Clin Ther 38(7):1589–1599
Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z et al (2018) ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med 24(5):556–562
Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their contribution to the evaluation of immunohistochemistry results and excellent pathological technology help.
Funding
This study was funded by grants from National Natural Science Foundation of China (81671628, 81871306, 81871926, 81871930, 81902402, 81902901, 81972219), Shanghai Municipal Natural Science Foundation (18ZR1432900), and Shanghai Sailing Program (17YF1402200, 18YF1404600, 19YF1407500). All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Author information
Authors and Affiliations
Contributions
M. Shi, Y. Gu, K. Jin and H. Fang for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; Y. Chen, Y. Cao, X. Liu, K. Lv, X. He, C. Lin, H. Liu, H. Li, H. He and J. Qin for technical and material support; R. Li, H. Zhang and W. Zhang for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The protocol of all study was approved by the institutional review board and ethics committee of Zhongshan Hospital, Fudan University.
Informed consent
Written informed consent was obtained from each patient included.
Consent for publication
All authors provide their consent for publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, M., Gu, Y., Jin, K. et al. CD47 expression in gastric cancer clinical correlates and association with macrophage infiltration. Cancer Immunol Immunother 70, 1831–1840 (2021). https://doi.org/10.1007/s00262-020-02806-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02806-2