Abstract
Purpose
This study was designed to investigate the correlation between immune-related adverse events (irAEs) of immune checkpoint inhibitors (ICIs) and corresponding efficacy, and to explore the potential of predicting the efficacy of ICIs via irAEs.
Methods
Electronic databases including PubMed, Embase, Cochrane Library, CNKI and Wanfang were applied to search for relevant studies. The primary endpoint was overall survival (OS) or progression-free survival (PFS), and the secondary endpoint was objective response rate (ORR). Stratification analyses were conducted according to the type of irAEs and ICIs, region of studies and primary tumors. Furthermore, statistical analyses were realized by means of RevMan 5.3 software.
Results
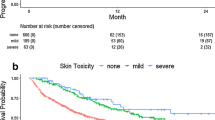
Altogether, 40 studies with 8,641 participants were enrolled, among which the incidence of irAEs ranged from 15.34 to 85.23% and the major sites reached out to skin, endocrine organ, gastrointestinal tract, liver and lung. The ORR, OS and PFS in irAE group were significantly higher than those in non-irAE group as per pooled analyses and stratification analyses. Importantly, patients with irAEs in skin, endocrine organ or gastrointestinal tract rather than in liver and lung were found to obtain survival benefits (p < 0.05).
Conclusion
IrAEs, especially in skin, endocrine organ or gastrointestinal tract, triggered by ICIs indicate significant survival benefits.





Similar content being viewed by others
References
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L et al (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. https://doi.org/10.1056/NEJMoa1412082
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372(26):2521–2532. https://doi.org/10.1056/NEJMoa1503093
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813. https://doi.org/10.1056/NEJMoa1510665
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L et al (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026. https://doi.org/10.1056/NEJMoa1613683
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375(19):1856–1867. https://doi.org/10.1056/NEJMoa1602252
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A et al (2016) Pembrolizumab versus chemotherapy for PD-L1 positive non-small-cell lung Cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349):409–413. https://doi.org/10.1126/science.aan6733
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA et al (2017) Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18(9):1182–1191. https://doi.org/10.1016/S1470-2045(17)30422-9
El-Khoueiry AB, Sangro B, Yau T, Crocenzio TS, Kudo M, Hsu C et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M et al (2018) Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 4(5):e180013. https://doi.org/10.1001/jamaoncol.2018.0013
Ito A, Kondo S, Tada K, Kitano S (2015) Clinical development of immune checkpoint inhibitors. Biomed Res Int 2015:605478. https://doi.org/10.1155/2015/605478
Xu YY, Wan B, Chen X, Zhan P, Zhao Y, Zhang T et al (2019) The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res 8(4):413–428
Cao DD, Xu HL, Xu XM, Guo T, Ge W (2019) High tumor mutation burden predicts better efficacy of immunotherapy: a pooled analysis of 103078 cancer patients. Onconimmunology 8(9):e1629258. https://doi.org/10.1080/2162402X.2019.1629258
Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22(4):886–894. https://doi.org/10.1158/1078-0432.CCR-15-1136
Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI et al (2015) Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33(7):773–781. https://doi.org/10.1200/JCO.2014.57.4756
Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152(1):45–51. https://doi.org/10.1001/jamadermatol.2015.2707
Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S et al (2017) Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol 44(2):117–122. https://doi.org/10.1111/1346-8138.13520
Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T et al (2019) High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer 10(10):2006–2012. https://doi.org/10.1111/1759-7714.13187
Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S (2013) Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 8(12):e83138. https://doi.org/10.1371/journal.pone.0083138
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR et al (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. https://doi.org/10.1186/1745-6215-8-16
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M et al (2014) Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med 12:116. https://doi.org/10.1186/1479-5876-12-116
Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M et al (2019) Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer 20(4):237–247. https://doi.org/10.1016/j.cllc.2019.02.006
Elias R, Yan F, Singla N, Levonyack N, Formella J, Christie A et al (2019) Immune-related adverse events are associated with improved outcomes in ICI-treated renal cell carcinoma patients. J Clin Oncol 37(7):S645
Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N et al (2019) Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer 20(3):201–207. https://doi.org/10.1016/j.cllc.2018.10.002
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4(3):374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK et al (2015) Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 33(28):3193–3198. https://doi.org/10.1200/JCO.2015.60.8448
Indini A, Guardo LD, Cimminiello C, Prisciandaro M, Randon G, Braud FD et al (2019) Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 145(2):511–521. https://doi.org/10.1007/s00432-018-2819-x
Kawai T, Sato Y, Makino K, Yamada Y, Nomiya A, Nakamura M et al (2019) Immune-related adverse events predict the therapeutic efficacy of pembrolizumab in urothelial cancer patients. Eur J Cancer 116:114–115. https://doi.org/10.1016/j.ejca.2019.05.017
Masuda K, Shoji H, Nagashima K, Yamamoto S, Ishikawa M, Imazeki H et al (2019) Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 19(1):974. https://doi.org/10.1186/s12885-019-6150-y
Okada N, Kawazoe H, Takechi K, Matsudate Y, Utsunomiya R, Zamami Y et al (2019) Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: a multicenter retrospective study. Clin Ther 41(1):59–67. https://doi.org/10.1016/j.clinthera.2018.11.004
Okamoto I, Sato H, Kondo T, Koyama N, Fushimi C, Okada T et al (2019) Efficacy and safety of nivolumab in 100 patients with recurrent or metastatic head and neck cancer - a retrospective multicentre study. Acta Otolaryngol 139(10):918–925. https://doi.org/10.1080/00016489.2019.1648867
Ricciuti B, Genova C, Giglio AD, Bassanelli M, Giovanna MDB, Metro G et al (2019) Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 145(2):479–485. https://doi.org/10.1007/s00432-018-2805-3
Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A et al (2019) Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer 109:21–27. https://doi.org/10.1016/j.ejca.2018.10.014
Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A et al (2018) Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74. https://doi.org/10.1016/j.lungcan.2017.11.019
Shafqat H, Gourdin T, Sion A (2018) Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy. Semin Oncol 45(3):156–163. https://doi.org/10.1053/j.seminoncol.2018.07.003
Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y et al (2017) Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol 12(12):1798–1805. https://doi.org/10.1016/j.jtho.2017.08.022
Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T et al (2019) Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol 5(3):376–383. https://doi.org/10.1001/jamaoncol.2018.5860
Verzoni E, Cartenì G, Cortes E, Giannarelli D, Giglio AD, Sabbatin R et al (2019) Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer 7(1):99. https://doi.org/10.1186/s40425-019-0579-z
Von Pawel J, Syrigos K, Mazieres J, Cortinovis D, Dziadziuszko R, Gandara DR, et al (2017) Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: analyses from the phase III study OAK. Ann Oncol 28(suppl 5): mdx380.017-mdx380.017
Bjørnhart B, Hansen KH, Jørgensen TL, Herrstedt J, Schytte T (2019) Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: a retrospective cohort study. Acta Oncol 58(7):953–961. https://doi.org/10.1080/0284186X.2019.1615636
Otsuka M, Sugihara S, Mori S, Hamada K, Sasaki Y, Yoshikawa S et al (2020) Immune-related adverse events correlate with improved survival in patients with advanced mucosal melanoma treated with nivolumab: a single-center retrospective study in Japan. J Dermatol 47(4):356–362. https://doi.org/10.1111/1346-8138.15246
Dick J, Lang N, Slynko A, Kopp-Schneider A, Schulz C, Dimitrakopoulo-strauss A et al (2016) Use of LDH and autoimmune side effects to predict response to ipilimumab treatment. Immunotherapy 8(9):1033–1044. https://doi.org/10.2217/imt-2016-0083
Judd J, Ma Z, Handorf E, O’Neill J, Ramamurthy C, Bentota S et al (2017) Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist 22(10):1232–1237. https://doi.org/10.1634/theoncologist.2017-0133
Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H et al (2017) Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology 7(1):e1375642. https://doi.org/10.1080/2162402X.2017.1375642
Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E, Poonja Z et al (2018) Efficacy of nivolumab and pembrolizumab in patients with advanced nonsmall cell lung cancer needing treatment interruption due to adverse events: aretrospective multicenter analysis. Clin Lung Cancer 20(1):e97–e106. https://doi.org/10.1016/j.cllc.2018.09.005
Lesueur P, Escande A, Thariat J, Vauléon E, Monnet I, Cortot A et al (2018) Safety of combined PD-1 pathway inhibition and radiation therapy for non-small-cell lung cancer: A multicentric retrospective study from the GFPC. Cancer Med 7(11):5505–5513. https://doi.org/10.1002/cam4.1825
Lisberg A, Tucker DA, Goldman JW, Wolf B, Carroll J, Ariana H et al (2018) Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res 6(3):288–294. https://doi.org/10.1158/2326-6066.CIR-17-0063
Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M et al (2019) Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol 37(30):2730–2737. https://doi.org/10.1200/JCO.19.00318
Min Lee CK, Li S, Tran DC, Zhu GA, Kim J, Kwong BY et al (2018) Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol 79(6):1047–1052. https://doi.org/10.1016/j.jaad.2018.05.035
Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K et al (2016) Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 7(47):77404–77415
Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D et al (2017) Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 28(3):583–589. https://doi.org/10.1093/annonc/mdw640
Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K et al (2018) Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer 19(6):e893–e900. https://doi.org/10.1016/j.cllc.2018.08.008
Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA et al (2015) Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 151(11):1206–1212. https://doi.org/10.1001/jamadermatol.2015.1916
Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N et al (2020) Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-small-cell lung cancer. Thorac Cancer 11(4):1052–1060. https://doi.org/10.1111/1759-7714.13364
Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM et al (2018) Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 67(3):459–470. https://doi.org/10.1007/s00262-017-2092-x
Weber JS, Hodi FS, Wolchok JD, Topalian SL et al (2017) Safety profile of nivolumab monotherapy: apooled analysis of patients with advanced melanoma. J Clin Oncol 35(7):785–792. https://doi.org/10.1200/JCO.2015.66.1389
Yamazaki N, Kiyohara Y, Uhara H, Uehara J, Fujimoto M, Takenouchi T et al (2017) Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a Phase II study. Cancer Sci 108(6):1223–1230. https://doi.org/10.1111/cas.13241
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168. https://doi.org/10.1056/NEJMra1703481
Patil PD, Fernandez AP, Velcheti V, Tarhini A, Funchain P, Rini B et al (2019) Cases from the irAE tumor board: a multidisciplinary approach to a patient treated with immune checkpoint blockade who presented with a new rash. Oncologist 24(1):4–8. https://doi.org/10.1634/theoncologist.2018-0434
Le Poole IC, Wañkowicz-Kaliñska A, van den Wijngaard RM, Nickoloff BJ, Das PK (2004) Autoimmune aspects of depigmentation in vitiligo. J Investig Dermatol Symp Proc 9(1): 68–72. https://doi.org/https://doi.org/10.1111/j.1087-0024.2004.00825.x
Abu-Sbeih H, Ali FS, Qiao W, Lu Y, Patel S, Diab A et al (2019) Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother 68(4):553–561. https://doi.org/10.1007/s00262-019-02303-1
Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C et al (2017) Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 28(6):1368–1379. https://doi.org/10.1093/annonc/mdx108
Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L et al (2016) Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 7:10391. https://doi.org/10.1038/ncomms10391
Osta BE, Hu F, Sadek R, Chintalapally R, Tang SC (2017) Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 119:1–12. https://doi.org/10.1016/j.critrevonc.2017.09.002
Wang DY, Salem J, Cohen JV, Chandra S, Menzer C, Fi Ye et al (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4(12):1721–1728. https://doi.org/10.1001/jamaoncol.2018.3923
Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L (2007) Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 7(2):95–106. https://doi.org/10.1038/nrc2051
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14(12):1212–1218. https://doi.org/10.1038/ni.2762
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L et al (2020) Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11(2):155–164. https://doi.org/10.1016/S1470-2045(09)70334-1
Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U (2019) Rheumatic syndromes associated with immune-checkpoint inhibitors: a single-center cohort of 61 patients. Arthritis Rheumatol 71(3):468–475. https://doi.org/10.1002/art.40745
Zhou XX, Yao ZR, Yang HX, Liang NX, Zhang X, Zhang FC (2020) Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 18(1):87–100. https://doi.org/10.1186/s12916-020-01549-2
Funding
This work was supported by the Natural Science Foundation of Fujian Province (2019J01457).
Author information
Authors and Affiliations
Contributions
Conceptualization: XX and LZ; methodology: LZ and JL; software: LZ; validation: all authors; formal analysis: LZ, FC and XX; data curation: LZ; writing—original draft preparation: LZ, QW and XX; writing—review and editing: all authors; visualization: LZ; supervision: XX.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, L., Wu, Q., Chen, F. et al. Immune-related adverse events: promising predictors for efficacy of immune checkpoint inhibitors. Cancer Immunol Immunother 70, 2559–2576 (2021). https://doi.org/10.1007/s00262-020-02803-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02803-5