Abstract
Background
The objective of this study was to investigate the association between the onset of TD and treatment efficacy in NSCLC patients who initiated anti-PD-1 blockade (Nivolumab®) and to assess the impact of TD severity and subtype on nivolumab efficacy.
Materials and methods
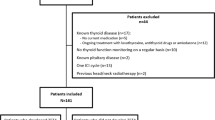
This study was performed at a referral oncology center between July 20, 2015 and June 30, 2018. Patients with histologically confirmed stage IIIB/IV NSCLC in progression after one or two lines of treatment and who initiated Nivolumab were included. Thyroid function (TSH ± fT4, fT3) was monitored and patients were classified according to TD status [TD(+) versus TD(−)], severity [moderate thyroid dysfunction: TSH level between 0.1 and 0.4 or 4.0 and 10 mIU/L and severe thyroid dysfunction: TSH ≤ 0.1 or ≥ 10mUI/L) and subtype (isolated hypothyroidism, isolated hyperthyroidism and hyperthyroidism then hypothyroidism)]. Clinical endpoints were overall survival (OS) and progression-free survival (PFS).
Results
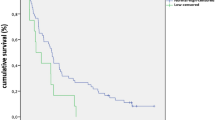
Among 194 eligible patients, 134 patients (median age, 63 yo; 70.1% male) were included. Forty (29.9%) patients were classified in TD(+) and had a longer OS of 29.8 months (95% CI 18.8-NR) versus 8.1 months (95% CI 5.5–11.5) in TD(−) group (p < 0.001). PFS was also longer (8.7 months (95% CI 5.3–15.1) in TD(+) versus 1.7 months (95% CI 1.6–1.9) in TD(−) group (p < 0.001). In Cox proportional hazards analysis, TD remained an independent predictive factor of OS/PFS. Severity and subtype of TD were not correlated with OS/PFS.
Conclusions
This study suggested that TD induced by Nivolumab appears to be an independent predictive factor of survival, irrespective of TD severity and subtype.



Similar content being viewed by others
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Brahmer J, Reckamp KL, Baas P et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
HAS, Commission de la transparence Avis du 2 février 2017 (relatif à l’AMM du Nivolumab 20/07/2015)
HAS, Commission de la transparence Avis du 11 janvier 2017 (relatif à l’AMM du Nivolumab 04/04/2016)
Byun DJ, Wolchok JD, Rosenberg LM, Girotra M (2017) Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 13(4):195–207. https://doi.org/10.1038/nrendo.2016.205
de Filette J, Andreescu C, Cools F, Bravenboer B, Velkeniers B (2019) A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res 51(03):145–156. https://doi.org/10.1055/a-0843-3366
Teulings H-E, Limpens J, Jansen SN et al (2015) Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33(7):773–781. https://doi.org/10.1200/JCO.2014.57.4756
Sanlorenzo M, Vujic I, Daud A et al (2015) Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 151(11):1206–1212. https://doi.org/10.1001/jamadermatol.2015.1916
Hua C, Boussemart L, Mateus C et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152(1):45–51. https://doi.org/10.1001/jamadermatol.2015.2707
Al Mushref M, Guido PA, Collichio FA, Moore DT, Clemmons DR (2019) Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors: a retrospective review. Endocr Pract 26(1):36–42. https://doi.org/10.4158/EP-2019-0244
Rizvi NA, Mazières J, Planchard D et al (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16(3):257–265. https://doi.org/10.1016/S1470-2045(15)70054-9
Gulley JL, Rajan A, Spigel DR et al (2017) Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 18(5):599–610. https://doi.org/10.1016/S1470-2045(17)30240-1
Herbst RS, Baas P, Kim D-W et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Osorio JC, Ni A, Chaft JE et al (2017) Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 28(3):583–589. https://doi.org/10.1093/annonc/mdw640
Ramos-Levi AM, Rogado J, Sanchez-Torres JM, Colomer R, Marazuela M (2019) Nivolumab-induced thyroid dysfunction in patients with lung cancer. Endocrinología Diabetes Nutrición 66(1):26–34. https://doi.org/10.1016/j.endinu.2018.05.005
Kim HI, Kim M, Lee S-H et al (2018) Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. OncoImmunology 7(1):e1375642. https://doi.org/10.1080/2162402X.2017.1375642
Yamauchi I, Yasoda A, Matsumoto S et al (2019) Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE 14(5):e0216954. https://doi.org/10.1371/journal.pone.0216954
Morganstein DL, Lai Z, Spain L et al (2017) Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol 86(4):614–620. https://doi.org/10.1111/cen.13297
Haanen JBAG, Carbonnel F, Robert C et al (2017) Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv119–iv142. https://doi.org/10.1093/annonc/mdx225
Illouz F, Drui D, Caron P, Do Cao C (2018) Expert opinion on thyroid complications in immunotherapy. Annales d’Endocrinologie. https://doi.org/10.1016/j.ando.2018.07.007
Castinetti F, Albarel F, Archambeaud F et al (2018) French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer 26(2):G1–G18. https://doi.org/10.1530/ERC-18-0320
Kerr KM, Hirsch FR (2016) Programmed death ligand-1 immunohistochemistry: friend or foe? Arch Pathol Lab Med 140(4):326–331. https://doi.org/10.5858/arpa.2015-0522-SA
Califano R, Lal R, Lewanski C et al (2018) Patient selection for anti-PD-1/PD-L1 therapy in advanced non-small-cell lung cancer: implications for clinical practice. Future Oncol 14(23):2415–2431. https://doi.org/10.2217/fon-2018-0330
Le DT, Durham JN, Smith KN et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349):409–413. https://doi.org/10.1126/science.aan6733
Mezquita L, Auclin E, Ferrara R et al (2018) Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 4(3):351. https://doi.org/10.1001/jamaoncol.2017.4771
Haratani K, Hayashi H, Chiba Y et al (2018) Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol 4(3):374. https://doi.org/10.1001/jamaoncol.2017.2925
Hasan Ali O, Diem S, Markert E et al (2016) Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. OncoImmunology 5(11):e1231292. https://doi.org/10.1080/2162402X.2016.1231292
Areses Manrique MC, Mosquera Martínez J, García González J et al (2018) Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res 7(3):404–415. https://doi.org/10.21037/tlcr.2018.04.03
Spigel D, Schwartzberg L, Waterhouse D et al (2017) P3.02c–026 Is nivolumab safe and effective in elderly and PS2 patients with non-small cell lung cancer (NSCLC)? Results of CheckMate 153: Topic: IT. J Thorac Oncol 12(1):S1287–S1288. https://doi.org/10.1016/j.jtho.2016.11.1821
Teraoka S, Fujimoto D, Morimoto T et al (2017) Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol 12(12):1798–1805. https://doi.org/10.1016/j.jtho.2017.08.022
Sato K, Akamatsu H, Murakami E et al (2018) Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74. https://doi.org/10.1016/j.lungcan.2017.11.019
Tison A, Quéré G, Misery L et al (2019) Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol 71(12):2100–2111. https://doi.org/10.1002/art.41068
Conner SC, Trinquart L (2019) Survivorship bias in analyses of immune checkpoint inhibitor trials. JAMA Oncol 5(8):1226–1226. https://doi.org/10.1001/jamaoncol.2019.1187
Audigier-Valette C, Pérol M, Barlesi F et al (2018) Caractéristiques des patients atteints de cancer bronchopulmonaire non à petites cellules (CBNPC) traités par nivolumab en condition de vie réelle : première analyse de l’étude EVIDENS (lung cancer patients treated with nivolumab : a longitudinal, prospective, observational, multicentric study). Rev Mal Respir 35:A104–A105. https://doi.org/10.1016/j.rmr.2017.10.229
Geier M, Descourt R, Corre R et al (2018) Real life second-line nivolumab in advanced non-small cell lung cancer: a French observational multicenter study of 259 patients (ABCT-IMMUNOBZH). Cancer Rep Rev. https://doi.org/10.15761/CRR.1000164
Lazzaro D, Price M, de Felice M, Di Lauro R (1991) The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113(4):1093–1104
Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA (1995) Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem 270(14):8108–8114. https://doi.org/10.1074/jbc.270.14.8108
Koyama J, Horiike A, Yoshizawa T et al (2019) Correlation between thyroid transcription factor-1 expression, immune-related thyroid dysfunction, and efficacy of anti-programmed cell death protein-1 treatment in non-small cell lung cancer. J Thorac Dis 11(5):1919–1928. https://doi.org/10.21037/jtd.2019.04.102
de Filette J, Jansen Y, Schreuer M et al (2016) Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 101(11):4431–4439. https://doi.org/10.1210/jc.2016-2300
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
VK, NR are the guarantor of the study. PT, CJ, NR designed the study. PT realized the analysis. PT, CJ, ZA drafted the manuscript. PT, CJ, NR, ZA did the interpretation of data. NR, ZA, GC, GQ, RD, VK revised the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thuillier, P., Joly, C., Alavi, Z. et al. Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunol Immunother 70, 2023–2033 (2021). https://doi.org/10.1007/s00262-020-02802-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02802-6