Abstract
Background
Classic Hodgkin lymphoma (cHL) is a lymphoid malignancy in which the microenvironment, where the neoplastic cells are immersed, contributes to the lymphomagenesis process. Epstein–Barr virus (EBV) presence also influences cHL microenvironment composition and contributes to pathogenesis. An increase in PDL1 expression in tumor cells and at the microenvironment was demonstrated in adult cHL. Therefore, our aim was to assess PD1/PDL1 pathway and EBV influence on this pathway in pediatric cHL, given that in Argentina, our group proved a higher incidence of EBV-associated pediatric lymphoma in children.
Methods
For that purpose, EBV presence was assessed by in situ hybridization, whereas PD1 and PDL1 expressions were studied by immunohistochemistry. PDL1 genetic alterations were analyzed by FISH, and survival was evaluated for PD1 and PDL1 expressions.
Results
EBV presence demonstrated no influence neither on PD1 expression at the microenvironment nor on PDL1 expression at HRS tumor cells. Unexpectedly, only 38% pediatric cHL displayed PDL1 genetic alterations by FISH, and no difference was observed regarding EBV presence. However, in EBV-related cHL cases, a higher number of PDL1 + cells were detected at the microenvironment.
Conclusion
Even though a high cytotoxic environment was previously described in EBV-related pediatric cHL, it might be counterbalanced by an immunoregulatory micro-environmental PDL1 + niche. This regulation may render a cytotoxic milieu that unsuccessfully try to eliminate EBV + Hodgkin Reed Sternberg tumor cells in pediatric patients.



Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Liu Y, Sattarzadeh A, Diepstra A, Visser L, van den Berg A (2014) The microenvironment in classical Hodgkin lymphoma: an actively shaped and essential tumor component. Semin Cancer Biol 24:15–22
Seifert M, Scholtysik R, Küppers R (2019) Origin and pathogenesis of B cell lymphomas. Methods Mol Biol 1956:1–33
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Wherry EJ, Ahmed R (2004) Memory CD8 T-cell differentiation during viral infection. J Virol 78:5535–5545
Jiang Y, Li Y, Zhu B (2015) T-cell exhaustion in the tumor microenvironment. Cell Death Dis 6:e1792
Bryan LJ, Gordon LI (2015) Blocking tumor escape in hematologic malignancies: the anti-PD-1 strategy. Blood Rev 29:25–32
Küppers R, Engert A, Hansmann ML (2012) Hodgkin lymphoma. J Clin Invest 122:3439–3447
Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S (2008) Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 93:193–200
Carreras J, Lopez-Guillermo A, Roncador G, Villamor N, Colomo L, Martinez A, Hamoudi R, Howat WJ, Montserrat E, Campo E (2009) High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 27:1470–1476
Muenst S, Hoeller S, Dirnhofer S, Tzankov A (2009) Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol 40:1715–1722
Vardhana S, Younes A (2016) The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica 101:794–802
Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, Armand P, Chapuy B, de Jong D, Hoppe RT, Neuberg DS, Rodig SJ, Shipp MA (2016) PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 34:2690–2697
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Carey CD, Gusenleitner D, Lipschitz M, Roemer MGM, Stack EC, Gjini E, Hu X, Redd R, Freeman GJ, Neuberg D, Hodi FS, Liu XS, Shipp MA, Rodig SJ (2017) Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 130:2420–2430
Patel SS, Weirather JL, Lipschitz M, Lako A, Chen PH, Griffin GK, Armand P, Shipp MA, Rodig SJ (2019) The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4-positive T cells that are PD-1-negative. Blood 134:2059–2069
Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, Sugita Y, Yufu Y, Choi I, Abe Y, Uike N, Nagafuji K, Okamura T, Akashi K, Takayanagi R, Shiratsuchi M, Ohshima K (2015) Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 126:2193–2201
Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, Neuberg D, Shipp MA (2012) Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 18:1611–1618
Kataoka K, Miyoshi H, Sakata S, Dobashi A, Couronné L, Kogure Y, Sato Y, Nishida K, Gion Y, Shiraishi Y, Tanaka H, Chiba K, Watatani Y, Kakiuchi N, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Sanada M, Onozawa M, Teshima T, Yoshiki Y, Ishida T, Suzuki K, Shimada K, Tomita A, Kato M, Ota Y, Izutsu K, Demachi-Okamura A, Akatsuka Y, Miyano S, Yoshino T, Gaulard P, Hermine O, Takeuchi K, Ohshima K, Ogawa S (2019) Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia 33:1687–1699
Saito R, Abe H, Kunita A, Yamashita H, Seto Y, Fukayama M (2017) Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1+ immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol 30:427–439
Shannon-Lowe C, Rickinson A (2019) The global landscape of EBV-associated tumors. Front Oncol 9:713
Shanbhag S, Ambinder RF (2018) Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin 68:116–132
De Matteo E, García Lombardi M, Preciado MV, Chabay P (2019) Changes in EBV association pattern in pediatric classic Hodgkin lymphoma from a single institution in argentina. Front Oncol 9:881
Chabay PA, Preciado MV (2013) EBV primary infection in childhood and its relation to B-cell lymphoma development: a mini-review from a developing region. Int J Cancer 133:1286–1292
Tan GW, Visser L, Tan LP, van den Berg A, Diepstra A (2018) The microenvironment in epstein-barr virus-associated malignancies. Pathogens 7:E40
Barros MH, Vera-Lozada G, Soares FA, Niedobitek G, Hassan R (2012) Tumor microenvironment composition in pediatric classical Hodgkin lymphoma is modulated by age and Epstein-Barr virus infection. Int J Cancer 131:1142–1152
Barros MH, Segges P, Vera-Lozada G, Hassan R, Niedobitek G (2015) Macrophage polarization reflects T cell composition of tumor microenvironment in pediatric classical Hodgkin lymphoma and has impact on survival. PLoS ONE 10:e0124531
Jimenez O, Barros MH, De Matteo E, Garcia Lombardi M, Preciado MV, Niedobitek G, Chabay P (2019) M1-like macrophage polarization prevails in young children with classic Hodgkin Lymphoma from Argentina. Sci Rep 9:12687
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375–2390
Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH (2017) PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med 214:895–904
Li L, Sun R, Miao Y, Tran T, Adams L, Roscoe N, Xu B, Manyam G, Tan X, Zhang H, Xiao M, Tzankov A, Visco C, Dybkaer K, Bhagat G, Tam W, Hsi E, van Krieken J, You H, Huh J, Ponzoni M, Ferreri A, Moller M, Piris M, Zhang M, Winter J, Medeiros L, Rassidakis G, Vaupel C, Li Y, Dakappagari N, Xu-Monette Z, Young K (2019) PD-1/PD-L1 expression and interaction by automated quantitative immunofluorescent analysis show adverse prognostic impact in patients with diffuse large B-cell lymphoma having T-cell infiltration: a study from the International DLBCL Consortium Program. Mod Pathol 32:741–754
Li-Yang Hu, Xiao-Lu Xu, Rao H-L, Chen J, Lai R-C, Huang H-Q, Jiang W-Q, Lin T-Y, Xia Z-J, Cai Q-Q (2017) Expression and clinical value of programmed cell death-ligand 1 (PD-L1) in diffuse large B cell lymphoma: a retrospective study. Chin J Cancer 36:94
Wang Y, Wenzl K, Manske M, Asmann Y, Sarangi V, Greipp P, Krull J, Hartert K, He R, Feldman A, Maurer M, Slager S, Nowakowski G, Habermann T, Witzig T, Link B, Ansell S, Cerhan J, Novak A (2019) Amplification of 9p24.1 in diffuse large B-cell lymphoma identifies a unique subset of cases that resemble primary mediastinal large B-cell lymphoma. Blood Cancer J 9:73
Dilly-Feldis M, Aladjidi N, Refait J, Parrens M, Ducassou S, Rullier A (2019) Expression of PD-1/PD-L1 in children’s classical Hodgkin lymphomas. Pediatr Blood Cancer 66:e27571
Cohen M, Vistarop AG, Huaman F, Narbaitz M, Metrebian F, De Matteo E, Preciado MV, Chabay PA (2017) Cytotoxic response against Epstein Barr virus coexists with diffuse large B-cell lymphoma tolerogenic microenvironment: clinical features and survival impact. Sci Rep 7:10813
Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T, Uchiyama T (2008) PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 111:3220–3224
Vistarop AG, Cohen M, Huaman F, Irazu L, Rodriguez M, De Matteo E, Preciado MV, Chabay PA (2018) The interplay between local immune response and Epstein-Barr virus-infected tonsillar cells could lead to viral infection control. Med Microbiol Immunol 207:319–327
Lambert S, Martinez O (2007) Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol 179:8225–8234
Liu W, Shipp M (2017) Signaling pathways and immune evasion mechanisms in classical Hodgkin lymphoma. Blood 130:2265–2270
Sun Z, Fourcade J, Pagliano O, Chauvin JM, Sander C, Kirkwood JM, Zarour HM (2015) IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res 75:1635–1644
Cristino A, Nourse J, West R, Sabdia M, Law S, Gunawardana J, Vari F, Mujaj S, Thillaiyampalam G, Snell C, Gough M, Keane C, Gandhi M (2019) EBV microRNA-BHRF1-2-5p Targets the 3’UTR of immune checkpoint ligands PD-L1 and PD-L2. Blood 134:2261–2270
Veloza L, Teixido C, Castrejon N, Climent F, Carrió A, Marginet M, Soldini D, González-Farré B, Ribera-Cortada I, Lopez-Guillermo A, González-Barca C, Sierra A, Herrera M, Gómez C, Garcia A, Balagué O, Campo E, Martinez A (2019) Clinicopathological evaluation of the programmed cell death 1 (PD1)/programmed cell death-ligand 1 (PD-L1) axis in post-transplant lymphoproliferative disorders: association with epstein-barr virus, PD-L1 copy number alterations, and outcome. Histopathology 75:799–812
Acknowledgements
The authors thank Barbara Cao; Silvana Romero; Cristina Pabes and Maria Jose Andrade (Histopathological Laboratory, at the Ricardo Gutierrez Children’s Hospital) for their helpful histotechnical work.
Funding
This study was supported in part by grants from National Agency for Science and Technology Promotion (PICT 2015 n° 1533- PID clinic n°048- PICT 2017 1554). CP, DME and PMV are members of the CONICET Research Career Program. JO is a CONICET doctoral fellow.
Author information
Authors and Affiliations
Contributions
CP conceived of the study and its design, drafted the manuscript and acquired funding. JO and CS performed the experiments, the analysis and interpretation of data. GLM, DME and PMV participated in its coordination and modification. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Institutional guidelines regarding human experimentation were followed, according to the Helsinki Declaration of 1975. The protocol was approved by the Ethical Committee of Ricardo Gutierrez Children Hospital, and written informed assent and consent was obtained from all patients or patient´s parents depending on age.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
262_2020_2787_MOESM1_ESM.pdf
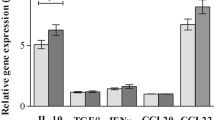
Supplementary Fig. 1: Event-free survival (EFS) analysis to IL10 + cells at microenvironment (cut off 50th percentile) (A) and TGFβ + cells at the microenvironment (cut off 50th percentile) (B) (PDF 70 KB)
Rights and permissions
About this article
Cite this article
Jimenez, O., Colli, S., Garcia Lombardi, M. et al. Epstein–Barr virus recruits PDL1-positive cells at the microenvironment in pediatric Hodgkin lymphoma. Cancer Immunol Immunother 70, 1519–1526 (2021). https://doi.org/10.1007/s00262-020-02787-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02787-2