Abstract
Introduction
A proteomic analysis of hepatocellular carcinoma (HCC) has revealed that Heat Shock Protein 70 (HSP70) is among the cancer antigen proteins of HCC. Moreover, we confirmed that HSP70 was highly expressed in HCC by immunohistochemical staining. Based on these results, we developed an HSP70 mRNA-transfected dendritic cell (DC) therapy for treating unresectable or recurrent HCC, and the phase I trial was completed successfully. Thus, we aimed to investigate the safety and efficacy of this therapy as a postoperative adjuvant treatment after curative resection for HCC to prevent recurrence by conducting a phase I/II randomized controlled clinical trial.
Methods
Patients (n = 45) with resectable HCC of stages II–IVa were registered and randomly assigned into two groups (DC group: 31 patients, control group: 14 patients) before surgery. The primary endpoint was disease-free survival (DFS), and the secondary endpoints were safety and overall survival. The DC therapy was initially administered at approximately 1 week after surgery, and twice every 3–4 weeks thereafter.
Results
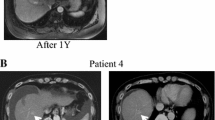
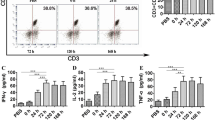
No adverse events specific to the immunotherapy were observed in the DC group. There was no difference in DFS between the DC and control groups (p = 0.666). However, in the subgroup with HSP70-expressing HCC, DFS of the DC group tended to be better (p = 0.090) and OS of the DC group was significantly longer (p = 0.003) than those of the control group.
Conclusion
The HSP70 mRNA-transfected DC therapy was performed safely as an adjuvant therapy. The prognosis of HSP70-expressing HCC cases could be expected to improve with this therapy.



Similar content being viewed by others
Change history
05 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00262-020-02819-x
References
Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J (2018) Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol 48:103–114
Ban D, Ogura T, Akahoshi K, Tanabe M (2018) Current topics in the surgical treatments for hepatocellular carcinoma. Ann Gastroenterol Surg 2:137–146
Bruix J, Takayama T, Mazzaferro V et al (2015) Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 16:1344–1354
Nishida N, Kudo M (2018) Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol Res 48(8):622–634
Hazama S, Tamada K, Yamaguchi Y, Kawakami Y, Nagano H (2018) Current status of immunotherapy against gastrointestinal cancers and its biomarkers: Perspective for precision immunotherapy. Ann Gastroenterol Surg 2(4):289–303
Matsui H, Hazama S, Tamada K et al (2019) Identification of a promiscuous epitope peptide derived from HSP70. J Immuno 42:244
Nakajima M, Hazama S, Tamada K et al (2020) A phase I study of multi-HLA-binding peptides derived from heat shock protein 70/glypican-3 and a novel combination adjuvant of hLAG-3Ig and Poly-ICLC for patients with metastatic gastrointestinal cancers: YNP01 trial. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-020-02518-7
Takayama T, Sekine T, Makuuchi M et al (2000) Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. The Lancet 356:802–807
Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, Toda T, Sakaida I, Okita K, Oka M, Nakamura K (2003) Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics 3:2487–2493. https://doi.org/10.1002/pmic.200300621
Yoshida S, Hazama S, Tokuno K, Sakamoto K, Takashima M, Tamesa T, Torigoe T, Sato N, Oka M (2009) Concomitant overexpression of heat-shock protein 70 and HLA class-I in hepatitis C virus-related hepatocellular carcinoma. Anticancer Res 29:539–544
Maeda Y, Yoshimura K, Matsui H et al (2015) Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol Immunother 64:1047–1056. https://doi.org/10.1007/s00262-015-1709-1
Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, Makuuchi M (2001) A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer 92:2374–2383
Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T, Kajiwara T (2000) Suppression of cellular immunity by surgical stress. Surgery 127:329–336
Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Bt T, Moutardier V, Farges O, Valla D (2001) Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 35:254–258
Huang G-T, Sheu J-C, Yang P-M, Lee H-S, Wang T-H, Chen D-S (1996) Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma—a study based on 420 patients. J Hepatol 25:334–338
Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, Ohtomo K, Makuuchi M (1999) Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology 30:889–893
Chen MF (1999) Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol 14:1144–1149
Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D (2002) Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 94:2040–2046
Liao SH, Su TH, Jeng YM, Liang PC, Chen DS, Chen CH, Kao JH (2019) Clinical manifestations and outcomes of patients with sarcomatoid hepatocellular carcinoma. Hepatology 69:209–221
Hasegawa K, Kokudo N, Makuuchi M et al (2013) Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 58:724–729
Shin E, Ryu HS, Kim S-H, Jung H, Jang J-J, Lee K (2011) The clinicopathological significance of heat shock protein 70 and glutamine synthetase expression in hepatocellular carcinoma. J Sci 18:544–550
Sakon M, Umeshita K, Nagano H et al (2000) Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg 135:1456–1459
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Seminars in cancer biology Elsevier, Newyork
Limagne E, Euvrard R, Thibaudin M et al (2016) Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX–bevacizumab drug treatment regimen. Can Res 76:5241–5252
Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V (2015) Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 136:2352–2360
Gonda K, Shibata M, Ohtake T, Matsumoto Y, Tachibana K, Abe N, Ohto H, Sakurai K, Takenoshita S (2017) Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol Lett 14:1766–1774
Li J, Hou Y, Cai X-B, Liu B (2016) Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol 22:4034
Xia F, Wu L-L, Lau W-Y, Huan H-B, Wen X-D, Ma K-S, Li X-W, Bie P (2016) Adjuvant sorafenib after heptectomy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma patients. World J Gastroenterol 22:5384
Lai EC, Lo C-M, Fan S-T, Liu C-L, Wong J (1998) Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg 133:183–188
Ono T, Yamanoi A, Nazmy El Assal O, Kohno H, Nagasue N (2001) Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: Metaanalysis of three randomized controlled trials. Cancer 91:2378–2385
Sawada Y, Yoshikawa T, Ofuji K et al (2016) Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 5:e1129483
Shimizu K, Kotera Y, Aruga A et al (2014) Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma. Hum Vac Immunothera 10:970–976
Lee J-H, Tak WY, Lee Y et al (2017) Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology 6:e1328335
Lee JH, Lee J-H, Lim Y-S et al (2015) Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148(1383–91):e6
Butterfield LH, Ribas A, Dissette VB et al (2006) A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four α-fetoprotein peptides. Clin Cancer Res 12:2817–2825
Acknowledgments
The authors thank Ms. Akiko Sano and Mrs. Kaori Kaneyasu for their excellent technical assistance with this work. The authors also thank Dr. Kohei Sakai for the generation of HSP70 mRNA.
Funding
No relevant funding was used for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design, data interpretation and review of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest.
Ethics approval
The protocol was approved by the institutional review board of Yamaguchi University (IRB number: H24-40) and was registered with the UMIN Clinical Trials Registry (registration no. UMIN000010691). The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Informed consent
All patients provided written informed consent prior to undergoing any study-related procedure.
Consent for publication
Consent for publication was obtained from all patients. In addition, all authors agree with the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Immunotherapy targeting HSP70 as a tumor-associated antigen (TSA) using mRNA-transfected DC was safe and effective for curatively resected HSP70-expressing HCC. This study revealed HSP70 could be a TSA for the immunotherapy against HCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsui, H.M., Hazama, S., Nakajima, M. et al. Novel adjuvant dendritic cell therapy with transfection of heat-shock protein 70 messenger RNA for patients with hepatocellular carcinoma: a phase I/II prospective randomized controlled clinical trial. Cancer Immunol Immunother 70, 945–957 (2021). https://doi.org/10.1007/s00262-020-02737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02737-y