Abstract
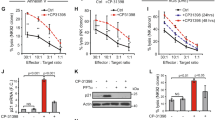
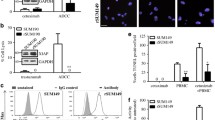
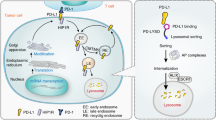
Among several mechanisms for the resistance of human epidermal growth factor receptor 2-overexpressing (HER2 +) cancer cells to trastuzumab, little is known regarding the mechanism underlying the resistance to trastuzumab-mediated antibody-dependent cellular cytotoxicity (ADCC). Cell death due to ADCC is caused by apoptosis of target cells induced by granzymes released from natural killer cells. Because optimal granzyme physiological activity occurs at neutral pH, we assumed that the pH of the intracellular environment influences the cytotoxic effects of granzymes. We established ADCC-resistant cells and compared them with wild-type cells in terms of the expression of intracellular pH-regulating genes. The expression of ATP6V1B1, which encodes a component of vacuolar ATPases, was downregulated in the ADCC-resistant cells. Thus, to functionally characterize ATP6V1B1, we used a CRISPR/Cas9 system to generate ATP6V1B1-knockout SKBR3 and JIMT-1 cells (both HER2 + human breast cancer cell line). The resulting cells exhibited significantly less ADCC than the control SKBR3 and JIMT-1 cells. The intracellular pH of the ATP6V1B1-knockout SKBR3 and JIMT-1 cells was significantly lower than control SKBR3 and JIMT-1cells. An analysis of granzyme dynamics during the ADCC reaction in cancer cells revealed that granzymes degraded intracellularly in the control SKBR3 and JIMT-1 cells and accumulated in ATP6V1B1-knockout cells, but were not cytotoxic. These findings suggest that decreased vacuolar ATPase activity alters the cytoplasmic pH of cancer cells to create an environment that is less suitable for granzyme bioactivity, which adversely affects the induction of apoptosis of cancer cells by NK cells.







Similar content being viewed by others
References
Slamon DJ et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792. https://doi.org/10.1056/NEJM200103153441101
Cameron D, Piccart-Gebhart MJ, Gelber RD et al (2017) 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389:1195–1205
Harari D, Yarden Y (2000) Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 19:6102–6114. https://doi.org/10.1038/sj.onc.1203973
Saez R et al (2006) p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res 12:424–431. https://doi.org/10.1158/1078-0432.CCR-05-1807
Scaltriti M et al (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99:628–638. https://doi.org/10.1093/jnci/djk134
Nagy P et al (2005) Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 65:473–482
Wang Y et al (2005) Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol Cancer Ther 4:1214–1221. https://doi.org/10.1158/1535-7163.MCT-05-0048
Nagata Y et al (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6(2):117–127. https://doi.org/10.1016/j.ccr.2004.06.022
Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 6:443–446
Reim F et al (2009) Immunoselection of breast and ovarian cancer cells with trastuzumab and natural killer cells: selective escape of CD44high/CD24low/HER2low breast cancer stem cells. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-09-0834
Scaltriti M et al (2009) Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. https://doi.org/10.1038/onc.2008.432
Roca L et al (2013) Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER-PACS 04 trial. Breast Cancer Res Treat 139(3):789–800. https://doi.org/10.1007/s10549-013-2587-x
Tamura K et al (2011) FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol 22:1302–1307
Thiery J et al (2011) Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol 12:770–777. https://doi.org/10.1038/ni.2050
Henkart PA, Berrebi GA, Takayama H, Munger WE, Sitkovsky MV (1987) Biochemical and functional properties of serine esterases in acidic cytoplasmic granules of cytotoxic T lymphocytes. J Immunol 139:2398–2405
Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA (2013) Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci 54:4330–4340. https://doi.org/10.1167/iovs.13-11929
Rousalova I, Krepela E (2010) Granzyme B-induced apoptosis in cancer cells and its regulation (review). Int J Oncol 37:1361–1378
Adrain C, Murphy BM, Martin SJ (2005) Molecular ordering of the caspase activation cascade initiated by the cytotoxic T lymphocyte/natural killer (CTL/NK) protease granzyme B. J Biol Chem 280:4663–4673
Martinvalet D, Dykxhoorn DM, Ferrini R, Lieberman J (2008) Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell 133:681–692
Breton S, Brown D (2013) Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda) 28:318–329. https://doi.org/10.1152/physiol.00007.2013
Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49:4715–4723
Arai S et al (2013) Rotation mechanism of Enterococcus hirae V1-ATPase based on asymmetric crystal structures. Nature 493:703–707. https://doi.org/10.1038/nature11778
van Hille B et al (1994) Heterogeneity of vacuolar H(+)-ATPase: differential expression of two human subunit B isoforms. Biochem J 303(Pt 1):191–198
Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11:50–61. https://doi.org/10.1038/nrm2820
Acknowledgements
This research was supported by a grant from the Japan Society for the Promotion of Science through the KAKENHI Grant Numbers 24591901. We thank Keiko Sakamoto, Yuji Fukushima, Masashi Inoue and Sunao Tanaka for teaching experimental techniques and helping with the experiments.
Funding
This research was supported by a grant from the Japan Society for the Promotion of Science (KAKENHI Grant Number 24591901).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests in the authorship or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nishie, M., Suzuki, E., Hattori, M. et al. Downregulated ATP6V1B1 expression acidifies the intracellular environment of cancer cells leading to resistance to antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother 70, 817–830 (2021). https://doi.org/10.1007/s00262-020-02732-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02732-3