Abstract
Objective
To assess the prognostic and predictive ability of early C-reactive protein (CRP) kinetics, dynamic changes in CRP levels, in patients with advanced urothelial cancer treated with pembrolizumab.
Patients and methods
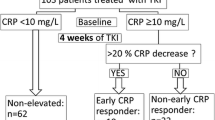
We retrospectively evaluated 97 patients with advanced urothelial cancer treated with pembrolizumab in second-line or later settings. Patients were divided into three early CRP kinetics groups: non-elevated (baseline CRP < 5 mg/L), responder (baseline CRP ≥ 5 mg/L and CRP decreased below baseline at least once within 30 days), and non-responder (baseline CRP ≥ 5 mg/L and CRP never decreased to baseline within 30 days). Association between early CRP kinetics and pembrolizumab efficacy including objective response rate (ORR), disease control rate (DCR), and overall survival (OS) were evaluated.
Results
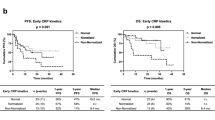
Based on early CRP kinetics, 40, 27, and 30 patients were classified as non-elevated, responder, and non-responder, respectively. ORR and DCR were 33% and 60% in non-elevated, 30% and 48% in responder, and 17% and 40% in non-responder; without a statistically significant difference. OS was significantly different among the non-elevated, responder, and non-responder groups (p < 0.01), with 1-year survival rates of 69%, 61%, and 31%, respectively. Early CRP kinetics could discriminate the OS of patients without objective response. Non-responder was an independent predictor for OS (HR 3.65, p < 0.01), as well as liver metastasis and ECOG PS ≥ 2.
Conclusion
Early CRP kinetics is associated with survival of advanced urothelial cancer patients treated with pembrolizumab and could be a potential biomarker for clinical benefit from immune checkpoint inhibitors.



Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- CR:
-
Complete response
- CRP:
-
C-reactive protein
- DCR:
-
Disease control rate
- ICI:
-
Immune checkpoint inhibitor
- iCPD:
-
Immune confirmed progressive disease
- irAE:
-
Immune related adverse event
- iUPD:
-
Immune undetermined progressive disease
- LDH:
-
Lactate dehydrogenase
- NLR:
-
Neutrophil to lymphocyte ratio
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PD-1:
-
Programmed cell death protein 1
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- SD:
-
Stable disease
References
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L et al (2017) Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026. https://doi.org/10.1056/NEJMoa1613683
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ et al (2019) Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol 30(6):970–976. https://doi.org/10.1093/annonc/mdz127
Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, Ascierto PA et al (2011) Defining the critical hurdles in cancer immunotherapy. J Transl Med 9:214. https://doi.org/10.1186/1479-5876-9-214
Kim C, Prasad V (2015) Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: An analysis of 5 years of US food and drug administration approvals. JAMA Inter Med 175(12):1992–1994. https://doi.org/10.1001/jamainternmed.2015.5868
Chiou VL, Burotto M (2015) Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 33(31):3541–3543. https://doi.org/10.1200/jco.2015.61.6870
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O et al (2016) Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 34(13):1510–1517. https://doi.org/10.1200/jco.2015.64.0391
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18(3):e143–e152. https://doi.org/10.1016/s1470-2045(17)30074-8
Roxburgh CS, McMillan DC (2010) Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 6(1):149–163. https://doi.org/10.2217/fon.09.136
Hilmy M, Bartlett JM, Underwood MA, McMillan DC (2005) The relationship between the systemic inflammatory response and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer 92(4):625–627. https://doi.org/10.1038/sj.bjc.6602406
Saito K, Kawakami S, Ohtsuka Y, Fujii Y, Masuda H, Kumagai J et al (2007) The impact of preoperative serum C-reactive protein on the prognosis of patients with upper urinary tract urothelial carcinoma treated surgically. BJU Int 100(2):269–273. https://doi.org/10.1111/j.1464-410X.2007.06934.x
Yoshida S, Saito K, Koga F, Yokoyama M, Kageyama Y, Masuda H et al (2008) C-reactive protein level predicts prognosis in patients with muscle-invasive bladder cancer treated with chemoradiotherapy. BJU Int 101(8):978–981. https://doi.org/10.1111/j.1464-410X.2007.07408.x
Gakis G, Todenhofer T, Renninger M, Schilling D, Sievert KD, Schwentner C et al (2011) Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: the TNR-C score. BJU Int 108(11):1800–1805. https://doi.org/10.1111/j.1464-410X.2011.10234.x
Ishioka J, Saito K, Sakura M, Yokoyama M, Matsuoka Y, Numao N et al (2012) Development of a nomogram incorporating serum C-reactive protein level to predict overall survival of patients with advanced urothelial carcinoma and its evaluation by decision curve analysis. Br J Cancer 107(7):1031–1036. https://doi.org/10.1038/bjc.2012.254
Morizane S, Iwamoto H, Yao A, Isoyama T, Sejima T, Takenaka A (2012) Serum C-reactive protein level is a significant prognostic indicator in patients with advanced urothelial cancer treated with gemcitabine-cisplatin or carboplatin - preliminary results. Cent European J Urol 65(2):62–66. https://doi.org/10.5173/ceju.2012.02.art1
Eggers H, Seidel C, Schrader AJ, Lehmann R, Wegener G, Kuczyk MA et al (2013) Serum C-reactive protein: a prognostic factor in metastatic urothelial cancer of the bladder. Med Oncol 30(4):705. https://doi.org/10.1007/s12032-013-0705-6
Casamassima A, Picciariello M, Quaranta M, Berardino R, Ranieri C, Paradiso A et al (2005) C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol 173(1):52–55. https://doi.org/10.1097/01.ju.0000146713.50673.e5
Tartour E, Blay JY, Dorval T, Escudier B, Mosseri V, Douillard JY et al (1996) Predictors of clinical response to interleukin-2–based immunotherapy in melanoma patients: a French multi-institutional study. J Clin Oncol 14(5):1697–1703. https://doi.org/10.1200/jco.1996.14.5.1697
Nyakas M, Aamdal E, Jacobsen KD, Guren TK, Aamdal S, Hagene KT et al (2019) Prognostic biomarkers for immunotherapy with ipilimumab in metastatic melanoma. Clin Exp Immunol 197(1):74–82. https://doi.org/10.1111/cei.13283
Heppt MV, Heinzerling L, Kahler KC, Forschner A, Kirchberger MC, Loquai C et al (2017) Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer 82:56–65. https://doi.org/10.1016/j.ejca.2017.05.038
Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N et al (2013) Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 24(6):1697–1703. https://doi.org/10.1093/annonc/mdt027
Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caraco C, Curvietto M et al (2014) Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 63(7):675–683. https://doi.org/10.1007/s00262-014-1545-8
Saito K, Tatokoro M, Fujii Y, Iimura Y, Koga F, Kawakami S et al (2009) Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol 55(5):1145–1153. https://doi.org/10.1016/j.eururo.2008.10.012
Saito K, Urakami S, Komai Y, Yasuda Y, Kubo Y, Kitsukawa S et al (2012) Impact of C-reactive protein kinetics on survival of patients with advanced urothelial carcinoma treated by second-line chemotherapy with gemcitabine, etoposide and cisplatin. BJU Int 110(10):1478–1484. https://doi.org/10.1111/j.1464-410X.2012.11153.x
Yasuda Y, Saito K, Yuasa T, Uehara S, Kawamura N, Yokoyama M et al (2017) Early response of C-reactive protein as a predictor of survival in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Int J Clin Oncol 22(6):1081–1086. https://doi.org/10.1007/s10147-017-1166-2
Suzuki K, Terakawa T, Furukawa J, Harada K, Hinata N, Nakano Y et al (2020) C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int J Clin Oncol 25(1):135–144. https://doi.org/10.1007/s10147-019-01528-5
Ozawa Y, Amano Y, Kanata K, Hasegwa H, Matsui T, Kakutani T et al (2019) Impact of early inflammatory cytokine elevation after commencement of PD-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol 36(4):33. https://doi.org/10.1007/s12032-019-1255-3
Sonpavde G, Manitz J, Gao C, Tayama D, Kaiser C, Hennessy D et al (2020) Five-factor prognostic model for survival of post-platinum patients with metastatic urothelial carcinoma receiving PD-L1 inhibitors. J Urol [published online ahead of print, 2020 Jun 18] 101097JU0000000000001199. https://doi.org/10.1097/JU.0000000000001199
Li M, Spakowicz D, Burkart J, Patel S, Husain M, He K et al (2019) Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol 145(10):2541–2546. https://doi.org/10.1007/s00432-019-02982-4
Powles T, Loriot Y, Gschwend JE, Bellmunt J, Geczi L, Vulsteke C et al (2018) KEYNOTE-361: Phase 3 trial of pembrolizumab ± chemotherapy versus chemotherapy alone in advanced urothelial cancer. Eur Urol Suppl 17:e1147–e1148. https://doi.org/10.1016/S1569-9056(18)31636-1
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Y.F. and N.M received honoraria for lectures from MSD K.K., a subsidiary of Merk & Co., Inc., Kenilworth, NJ, USA. All other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kijima, T., Yamamoto, H., Saito, K. et al. Early C-reactive protein kinetics predict survival of patients with advanced urothelial cancer treated with pembrolizumab. Cancer Immunol Immunother 70, 657–665 (2021). https://doi.org/10.1007/s00262-020-02709-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02709-2