Abstract
Objectives
Early clinical trials showed promising outcomes with immune-checkpoint inhibitors (ICI) in a subset of patients with relapsed small-cell lung carcinoma (SCLC). The aim of this retrospective analysis was to assess the efficacy and safety of ICI for relapsed SCLC in a real-world patient population.
Methods
Nine cancer centres in Switzerland contributed data to this cohort. Responses were assessed by the local investigators using standard RECIST v1.1 criteria. Progression-free survival (PFS) and overall survival (OS) were analysed by the Kaplan–Meier method. Associations between potential predictive markers and survival endpoints were probed by Cox proportional hazards.
Results
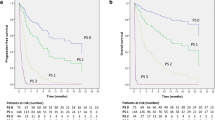
Forty-five patients were included in the analysis. Median age was 63 years, 73% were males and 18% had an ECOG performance status (PS) ≥ 2. ICIs were given as second-line treatment in 60%. Twenty-four patients (53%) received ipilimumab with nivolumab. Twenty-eight patients (62%) had undergone irradiation (RT) prior to or during ICI. Overall response rate (ORR) was 29% and median PFS and OS were 2.3 and 6.5 months, respectively. Median duration of response was 9 months (95% CI 2.8–NA). Five patients maintained their response for > 6 months, all of them receiving combination treatment. There were no new safety signals.
Conclusion
This is the first report of “real-world” data on ICI in relapsed SCLC also including patients with poor PS. Promising durable responses were observed. No biological prognostic marker could be identified.


Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- CPS:
-
Composite proportion score
- CR:
-
Complete remission
- CT:
-
Computed tomography
- DCR:
-
Disease-control rate
- DOR:
-
Duration of response
- ICI:
-
Immune checkpoint inhibitors
- irAE:
-
Immune-related adverse events
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- PD-L1:
-
Programmed death ligand 1
- PR:
-
Partial remission
- PS:
-
Performance status
- RECIST:
-
Response evaluation criteria in the solid tumours guidelines
- RT:
-
Radiotherapy
- SCLC:
-
Small-cell lung cancer
- SD:
-
Stable disease
- TMB:
-
Tumour mutational burden
- TPS:
-
Tumour proportion score
References
Pujol JL, Carestia L, Daures JP (2000) Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 83:8–15
Mascaux C, Paesmans M, Berghmans T et al (2000) A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 30:23–36
Horn L, Mansfield AS, Szczęsna A et al (2018) First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 379:2220–2229
Paz-Ares L (2019) PL02.11 Overall survival with durvalumab plus etoposide-platinum in first-line extensive-stage SCLC: results from the CASPIAN study. J Thoracic Oncol 14:S7–S8
Foster NR, Qi Y, Shi Q et al (2011) Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: findings on the basis of North Central Cancer Treatment Group trials. Cancer 117:1262–1271
O’Brien ME, Ciuleanu TE, Tsekov H et al (2006) Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 24:5441–5447
Eckardt JR, von Pawel J, Pujol JL et al (2007) Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 25:2086–2092
von Pawel J, Schiller JH, Shepherd FA et al (1999) Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17:658–667
Antonia SJ, Lopez-Martin JA, Bendell J et al (2016) Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17:883–895
Ott PA, Elez E, Hiret S et al (2017) Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 Study. J Clin Oncol 35:3823–3829
Chung H, Lopez-Martin J, Kao S et al (2018) Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol 36:8506
Ready N, Farago AF, de Braud F et al (2019) Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol 14:237–244
Reck M, Vincente D, Ciuleanu T et al (2018) LBA5—efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann Oncol 29:x39–x43
Pujol JL, Greillier L, Audigier-Valette C et al (2019) A randomized non-comparative phase 2 study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol 14:903–913
Chung H, Piha-Paul S, Lopez-Martin J et al (2019) CT073—Pembrolizumab after two or more lines of prior therapy in patients with advanced small-cell lung cancer (SCLC): results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 15(4):618–627
Goldman JW, Dowlati a, Antonia SJ et al (2018) Safety and antitumor activity of durvalumab monotherapy in patients with pretreated extensive disease small-cell lung cancer (ED-SCLC). J Clin Onco 36(15_suppl):8518–8518
Cho D, Mahipal A, Dowlati A et al (2018) Safety and clinical activity of durvalumab in combination with tremelimumab in extensive disease small-cell lung cancer (ED-SCLC). J Clin Oncol 36(15_suppl):8517–8517
Hellmann MD, Ott PA, Zugazagoitia J et al (2017) Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J Clin Oncol 35(15_suppl):8503–8503
Lai WCV, Rizvi H, Egger JV et al (2019) Real-world experience and molecular features of response to immune checkpoint blockade in patients with recurrent small cell lung cancer. J Clin Oncol 37(15_suppl):8556–855
Graus F, Dalmou J, Rene R et al (1997) Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol 15:2866–2872
Darnell RB and Posner JB (2003) Paraneoplastic syndromes involving the nervous system. N Engl J Med 349:1543–1554
Owonikoko TK, Kim HR, Govindan R et al (2019) Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): Results from the double-blind, randomized phase III CheckMate 451 study. Ann Oncol 30 (Supplement 2):ii77–ii80
Shaverdian N, Lisberg AE, Bornakyan K et al (2017) Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 18(7):895–903
Diem S, Kasenda B, Spain L et al (2016) Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 114:256–261
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830
Fehrenbacher L, Spira A, Ballinger M et al (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387:1837–1846
Herbst RS, Baas P, Kim DW et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Hellmann MD, Ciuleanu TE, Pluzanski A et al (2018) Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093–2104
Sabari JK, Leonardi GC, Shu CA et al (2018) PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol 29:2085–2091
Goodman AM, Kato S, Bazhenova L et al (2017) Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 16:2598–2608
Chan TA, Yarchoan M, Jaffee E et al (2019) Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 30:44–56
Daud AI, Loo K, Pauli ML et al (2016) Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest 126:3447–3452
Schmid S, Fruh M (2018) Immune checkpoint inhibitors and small cell lung cancer: what’s new? J Thorac Dis 10:S1503–S1508
Schultheis AM, Scheel AH, Ozretic L et al (2015) PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 51:421–426
George J, Saito M, Tsuta K et al (2017) Genomic amplification of CD274 (PD-L1) in small-cell lung cancer. Clin Cancer Res 23:1220–1226
Yasuda Y, Ozasa H, Kim YH (2018) PD-L1 expression in small cell lung cancer. J Thorac Oncol 13:e40–e41
Ricciuti B, Kravets S, Dahlberg SE et al (2019) Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer 7:87
Ready NE, Ott PA, Hellmann MD et al (2019) Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol 15(3):426–435
Hellmann MD, Callahan MK, Awad MM et al (2018) Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 33:853–861
Acknowledgements
Institutional grant was received by BMS for molecular analysis.
Author information
Authors and Affiliations
Contributions
SS, LM and MF conceived and designed the study. SS, LM, AF, VB, SR, HB, PF, CB, DK, LW, WJ and AA collected and assembled the data. Data analysis and interpretation were done by the following authors: clinical data: SS, LM, SS (SämiSchär), MF; pathology: ID, WJ. SS, LM and MF were involved in overall interpretation. All authors were involved in manuscript writing. All authors gave the final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SS as the corresponding author and MF declare having received institutional funding from BMS for extended molecular analysis of this study. SS: grants (institutional): BMS, Astra Zeneca; honoraries for advisory boards (to the institution): Boehringer-Ingelheim, MSD; travel supplemort: MSD, Boehringer-Ingelheim, Takeda. LM: personal fees: Bristol Myers Squibb, AstraZeneca, Roche, Takeda, Merck Sharp and Dohme, Pfizer; non-financial support: Bristol Myers Squibb, AstraZeneca, Roche, Takeda, Merck Darmstadt. AF: personal fees: Roche, Pfizer, Astellas, BMS. SR: Grants: AstraZeneca, BMS, Merck Serono; honoraries for advisory boards (to the institution): AstraZeneca, BMS, Merck Serono, MSD, Roche, Novartis, Eli Lilly, Boehringer-Ingelheim, Eisai, Takeda, Bayer, Pfizer; serves as scientific advisor of the Federal Drug Commission of the Federal Office of Public Health. CB: consulting or advisory role: AstraZeneca, Pfizer, Roche, Takeda, Janssen-Cilag, Boehringer-Ingelheim; travel, accommodations/expenses: AstraZeneca, Takeda. AA: personal fees: BMS, Astra Zeneca, MSD, Takeda, Pfizer, Roche, Boehringer-Ingelheim; grants: Boehringer-Ingelheim. MF: grants BMS, Astra Zeneca (all institutional); honoraries for advisory boards (to the institution): BMS, MSD, Astra Zeneca, Boehringer-Ingelheim, Roche, Takeda.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supp. Figure A
PFS in patients with good versus poor performance status at start of ICI (PS 0–1 versus PS ≥ 2) (PDF 52 kb)
Supp. Figure B
OS in patients with good versus poor performance status at start of ICI (PS 0–1 versus PS ≥ 2) (PDF 59 kb)
Supp. Figure C
PFS in patients receiving ICI monotherapy versus combination therapy (PDF 63 kb)
Supp. Figure D
OS in patients receiving ICI monotherapy versus combination therapy (PDF 68 kb)
Supp. Figure E
PFS in patients with prior tumour irradiation (RT) versus patients without prior RT or prophylactic cranial irradiation (PCI) only (PDF 68 kb)
Supp. Figure F
OS in patients with prior tumour irradiation (RT) versus patients without prior RT or prophylactic cranial irradiation (PCI) only (PDF 73 kb)
Supp. Figure G
Correlation between TMB and response category. Horizontal lines show group median. (PDF 9 kb)
Supp. Figure H
PFS in patients with low (< 50th centile) versus high (> 50th centile) TMB (PDF 54 kb)
Supp. Figure J
OS in patients with low (< 50th centile) versus high (> 50th centile) TMB (PDF 55 kb)
Rights and permissions
About this article
Cite this article
Schmid, S., Mauti, L.A., Friedlaender, A. et al. Outcomes with immune checkpoint inhibitors for relapsed small-cell lung cancer in a Swiss cohort. Cancer Immunol Immunother 69, 1605–1613 (2020). https://doi.org/10.1007/s00262-020-02565-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02565-0