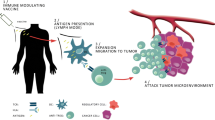
Abstract
l-arginine depletion by regulatory cells and cancer cells expressing arginase-1 (Arg-1) is a vital contributor to the immunosuppressive tumor microenvironment in patients with cancer. We have recently described the existence of pro-inflammatory effector T cells that recognize Arg-1. Hence, Arg-1-specific self-reactive T cells are a naturally occurring part of the memory T-cell repertoire of the human immune system. Here, we further characterize a highly immunogenic epitope from Arg-1. We describe frequent T-cell-based immune responses against this epitope in patients with cancer, as well as in healthy donors. Furthermore, we show that Arg-1-specific T cells expand in response to the TH2 cytokine interleukin (IL)-4 without any specific stimulation. Arg-1-specific memory TH1 cells that respond to increased IL-4 concentration may, therefore, drive the immune response back into the TH1 pathway. Arg-1-specific T cells thus appear to have an important function in immune regulation. Because Arg-1 plays an important role in the immunosuppressive microenvironment in most cancers, an immune modulatory vaccination approach can readily be employed to tilt the balance away from immune suppression in these settings.




Similar content being viewed by others
Abbreviations
- Arg1:
-
Arginase 1
- BC:
-
Breast cancer
- HD:
-
Healthy donor
- MM:
-
Malignant melanoma
- TAM:
-
Tumor-associated macrophages
- TNTC:
-
Too numerous to count
References
Geiger R et al (2016) l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167(3):829–842. https://doi.org/10.1016/j.cell.2016.09.031
Zea AH et al (2004) L-Arginine modulates CD3ζ expression and T cell function in activated human T lymphocytes. Cell Immunol 232(1–2):21–31. https://doi.org/10.1016/j.cellimm.2005.01.004
Rodriguez PC, Quiceno DG, Ochoa AC (2007) L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109(4):1568–1574. https://doi.org/10.1182/blood-2006-06-031856
De Boniface J, Mao Y, Schmidt-mende J, Kiessling R, Poschke I (2012) Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology 1(8):1305–1312. https://doi.org/10.4161/onci.21678
Lang S et al (2018) Clinical relevance and suppressive capacity of human MDSC subsets. Clin Cancer Res 24(19):4834–4844. https://doi.org/10.1158/1078-0432.CCR-17-3726
Rodriguez PC et al (2009) Arginase I—producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 69(4):1553–1561. https://doi.org/10.1158/0008-5472.CAN-08-1921
Rotondo R et al (2009) IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer 125:887–893. https://doi.org/10.1002/ijc.24448
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14(10):1014–1022. https://doi.org/10.1038/ni.2703
Andersen MH (2016) Anti-regulatory T cells. Semin Immunopathol. https://doi.org/10.1007/s00281-016-0593-x
Andersen MH (2015) Immune regulation by self-recognition: novel possibilities for anticancer immunotherapy. J Natl Cancer Inst 107(9):1–8. https://doi.org/10.1093/jnci/djv154
Andersen MH (2018) The balance players of the adaptive immune system. Cancer Res 15:1379–1383. https://doi.org/10.1158/0008-5472.CAN-17-3607
Martinenaite E et al (2018) Frequent adaptive immune responses against arginase-1. Oncoimmunology 7(3):1–9. https://doi.org/10.1080/2162402X.2017.1404215
Jørgensen MA et al (2018) Spontaneous T-cell responses against Arginase-1 in the chronic myeloproliferative neoplasms relative to disease stage and type of driver mutation. Oncoimmunology. https://doi.org/10.1080/2162402X.2018.1468957
Martinenaite E, Ahmad SM, Svane IM, Andersen MH (2019) Peripheral memory T cells specific for Arginase-1. Cell Mol Immunol. https://doi.org/10.1038/s41423-019-0231-3
Moodie Z, Price L, Janetzki S, Cedrik B (2012) Response determination criteria for ELISPOT: toward a standard that can be applied across laboratories. Methods Mol Biol 792:185–196. https://doi.org/10.1007/978-1-62703-239-1_1
Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 17(12):887–904. https://doi.org/10.1038/nrd.2018.169
Munder M, Eichmann K, Morán JM, Centeno F, Soler G, Modolell M (1999) Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol 163(7):3771–3777
Yu W, Jiang N, Quake SR, Davis MM (2015) Clonal deletion prunes but does not eliminate self-specific alphabeta CD8(+) T lymphocytes. Immunity 42:929–941. https://doi.org/10.1016/j.immuni.2015.05.001
Namdar A et al (2018) Prophylactic DNA vaccine targeting Foxp3 + regulatory T cells depletes myeloid-derived suppressor cells and improves anti-melanoma immune responses in a murine model. Cancer Immunol Immunother 67(3):367–379. https://doi.org/10.1007/s00262-017-2088-6
Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E (2007) Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res 67(1):371–380. https://doi.org/10.1158/0008-5472.CAN-06-2903
Munir S et al (2013) HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res 73(6):1764–1776. https://doi.org/10.1158/0008-5472.CAN-12-3507
Munir S, Andersen GH, Svane IM, Andersen MH (2013) The immune checkpoint regulator PD-L1 is a specific target for naturally occurring CD4(+) T cells. Oncoimmunology 2(4):e23991. https://doi.org/10.4161/onci.23991
Munir S, Andersen GH, Woetmann A, Ødum N, Becker JC, Andersen MH (2013) Cutaneous T cell lymphoma cells are targets for immune checkpoint ligand PD-L1-specific, cytotoxic T cells. Leukemia 27(11):2251–2253. https://doi.org/10.1038/leu.2013.118
Ahmad SM, Larsen SK, Svane IM, Andersen MH (2014) Harnessing PD-L1-specific cytotoxic T cells for anti-leukemia immunotherapy to defeat mechanisms of immune escape mediated by the PD-1 pathway. Leukemia 28(1):236–238. https://doi.org/10.1038/leu.2013.261
Soerensen RB, Hadrup SR, Svane IM, Hjortso MC, Straten PT, Andersen MH (2011) Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 117(7):2200–2210. https://doi.org/10.1182/blood-2010-06-288498
Andersen MH (2012) CD4 responses against IDO. Oncoimmunology 1(7):1211–1212. https://doi.org/10.4161/onci.20780
Larsen SK et al (2013) Functional characterization of Foxp3-specific spontaneous immune responses. Leukemia 27(12):2332–2340. https://doi.org/10.1038/leu.2013.196
Martinenaite E et al (2016) CCL22-specific T Cells: modulating the immunosuppressive tumor microenvironment. Oncoimmunology 5(11):e1238541. https://doi.org/10.1080/2162402X.2016.1238541
Funding
This work has been supported by grants from the Danish Cancer Society (Grant number R146-A9440-16-S2), Herlev Hospital (CCIT-Dk funding) and Innovation Fund Denmark (Grant number 8054-00058B).
Author information
Authors and Affiliations
Contributions
MHA designed and supervised the study. EM designed the experiments and analyzed the data. EM, SMA, SKB, MAJ, and SEWB performed experiments. IMS provided the relevant clinical material. All authors contributed to drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Mads Hald Andersen has filed several patent applications based on the use of arginase for vaccinations. The rights of the patent applications have been transferred to Copenhagen University Hospital, Herlev, in accordance with the Danish Law of Public Inventions at Public Research Institutions. The capital region has licensed these patents to the company IO Biotech ApS, whose purpose is to develop immune-modulating vaccines for cancer treatments. Mads Hald Andersen is a shareholder and board member of IO Biotech ApS. Evelina Martinenaite is employed by IO Biotech ApS. Other authors declare no conflict of interest.
Ethical approval and ethical standards
The protocol was approved by the Scientific Ethics Committee for the Capital Region of Denmark (H-A-2009-013) and conducted in accordance with the provisions of the Declaration of Helsinki.
Informed consent
Written informed consent for the use of the PBMCs for research purposes was obtained from the patients and healthy donors prior to inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is a Focussed Research Review based on a presentation given at the Eighteenth International Conference on Progress in Vaccination against Cancer (PIVAC 18), held in Oslo, Norway, 3rd–5th October, 2018. It is part of a Cancer Immunology, Immunotherapy series of PIVAC 18 papers.
Rights and permissions
About this article
Cite this article
Martinenaite, E., Ahmad, S.M., Bendtsen, S.K. et al. Arginase-1-based vaccination against the tumor microenvironment: the identification of an optimal T-cell epitope. Cancer Immunol Immunother 68, 1901–1907 (2019). https://doi.org/10.1007/s00262-019-02425-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02425-6