Abstract
Background
Immunotherapy has become a powerful treatment option for several solid tumor types. The presence of tumor-infiltrating lymphocytes (TIL) is correlated with better prognosis in ovarian cancer, pointing at the possibility to benefit from harnessing their anti-tumor activity. This preclinical study explores the feasibility of adoptive cell therapy (ACT) with TIL using an improved culture method.
Methods
TIL from high-grade serous ovarian cancer were cultured using a combination of IL-2 with agonistic antibodies targeting 4-1BB and CD3. The cells were phenotyped using flow cytometry in the fresh tissue and after expansion. Tumor reactivity was assessed against HLA-matched ovarian cancer cell lines via IFN-γ ELISPOT.
Results
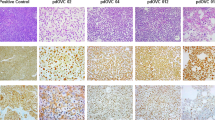
Ovarian cancer is highly infiltrated with CD8+ TIL that are preferentially and robustly expanded with the addition of the agonistic antibodies. With a 95% success rate, the TIL are grown to ≥ 100 × 106 cells in 2–3 weeks without over differentiation. In addition, the CD8+ TIL grown with this method showed HLA-restricted tumor recognition.
Conclusions
These results indicate the viability of TIL ACT for refractory ovarian cancer by allowing for the large expansion of anti-tumor TIL in a short time and consistent manner.




Similar content being viewed by others
Abbreviations
- BTLA:
-
B- and T-lymphocyte attenuator
- OvCa:
-
Epithelial ovarian cancer
- MDACC:
-
MD Anderson Cancer Center
- ORR:
-
Overall response rate
- TIL-CM:
-
Tumor-infiltrating lymphocyte complete medium
- T RM :
-
Tissue-resident memory T cell
References
Siegel RL, Miller KD (2018) Jemal A (2018) cancer statistics. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21442
Zsiros E, Tanyi J, Balint K, Kandalaft LE (2014) Immunotherapy for ovarian cancer: recent advances and perspectives. Curr Opin Oncol 26(5):492–500. https://doi.org/10.1097/CCO.0000000000000111
Vaughan S, Coward JI, Bast RC Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR (2011) Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11(10):719–725. https://doi.org/10.1038/nrc3144
Leitao MM, Chi DS (2009) Surgical management of recurrent ovarian cancer. Semin Oncol 36(2):106–111. https://doi.org/10.1053/j.seminoncol.2008.12.002
Ding M, Mei-jiao G (1991) Immune effect of tumor-infiltrating lymphocytes and its relation to the survival rate of patients with ovarian malignancies. J Tongji Med Univ 11(4):5
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348(3):203–213. https://doi.org/10.1056/NEJMoa020177
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102(51):18538–18543. https://doi.org/10.1073/pnas.0509182102
Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW (2009) Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother 58(3):449–459. https://doi.org/10.1007/s00262-008-0583-5
Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH (2012) CD20+ tumor-infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res 18(12):3281–3292. https://doi.org/10.1158/1078-0432.CCR-12-0234
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N (2013) Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 108(4):914–923. https://doi.org/10.1038/bjc.2013.32
Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H (2004) CD8+ Tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28(1):e26–e31. https://doi.org/10.1097/00006676-200401000-00023
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. https://doi.org/10.1056/NEJMoa1003466
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32(10):1020–1030. https://doi.org/10.1200/JCO.2013.53.0105
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. https://doi.org/10.1056/NEJMoa1504030
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369(2):122–133. https://doi.org/10.1056/NEJMoa1302369
Schachter J, Ribas A, Long GV, Arance A, Grob J-J, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C (2017) Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). The Lancet 390(10105):1853–1862. https://doi.org/10.1016/s0140-6736(17)31601-x
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, Rizvi NA (2016) Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 17(3):299–308. https://doi.org/10.1016/s1470-2045(15)00544-6
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813. https://doi.org/10.1056/NEJMoa1510665
Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G (2008) Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 105(8):3005–3010. https://doi.org/10.1073/pnas.0712237105
Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I (2015) Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33(34):4015–4022. https://doi.org/10.1200/JCO.2015.62.3397
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366(26):2455–2465. https://doi.org/10.1056/NEJMoa1200694
Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME (2011) Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17(13):4550–4557. https://doi.org/10.1158/1078-0432.CCR-11-0116
Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J (2010) Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 16(9):2646–2655. https://doi.org/10.1158/1078-0432.CCR-10-0041
Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, Glass M, Johnson VE, McMannis JD, Shpall E, Prieto V, Papadopoulos N, Kim K, Homsi J, Bedikian A, Hwu WJ, Patel S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Davies MA, Mansaray R, Fulbright OJ, Toth C, Ramachandran R, Wardell S, Gonzalez A, Hwu P (2012) Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res 18(24):6758–6770. https://doi.org/10.1158/1078-0432.CCR-12-1177
Forget MA, Haymaker C, Hess KR, Meng YJ, Creasy C, Karpinets T, Fulbright OJ, Roszik J, Woodman SE, Kim YU, Sakellariou-Thompson D, Bhatta A, Wahl A, Flores E, Thorsen ST, Tavera RJ, Ramachandran R, Gonzalez AM, Toth CL, Wardell S, Mansaray R, Patel V, Carpio DJ, Vaughn C, Farinas CM, Velasquez PG, Hwu WJ, Patel SP, Davies MA, Diab A, Glitza IC, Tawbi H, Wong MK, Cain S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Wargo JA, Radvanyi LG, Torres-Cabala CA, Beroukhim R, Hwu P, Amaria RN, Bernatchez C (2018) Prospective analysis of adoptive til therapy in patients with metastatic melanoma: response, impact of anti-CTLA4, and biomarkers to predict clinical outcome. Clin Cancer Res 24(18):4416–4428. https://doi.org/10.1158/1078-0432.CCR-17-3649
Aoki Y, Takakuwa K, Kodama S, Tanaka K, Takahashi M, Tokunaga A, Takahashi T (1991) Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian-cancer. Can Res 51(7):1934–1939
Freedman RS, Edwards CL, Kavanagh JJ, Kudelka AP, Katz RL, Carrasco CH, Atkinson EN, Scott W, Tomasovic B, Templin S et al (1994) Intraperitoneal adoptive immunotherapy of ovarian carcinoma with tumor-infiltrating lymphocytes and low-dose recombinant interleukin-2: a pilot trial. J Immunother Emphasis Tumor Immunol 16(3):198–210
Ikarashi H, Fujita K, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K (1994) Immunomodulation in patients with epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Cancer Res 54(1):190–196
Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K (1995) Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res 1(5):501–507
Freedman RS, Kudelka AP, Kavanagh JJ, Verschraegen C, Edwards CL, Nash M, Levy L, Atkinson EN, Zhang HZ, Melichar B, Patenia R, Templin S, Scott W, Platsoucas CD (2000) Clinical and biological effects of intraperitoneal injections of recombinant interferon-gamma and recombinant interleukin 2 with or without tumor-infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin Cancer Res 6(6):2268–2278
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26(32):5233–5239. https://doi.org/10.1200/JCO.2008.16.5449
Chacon JA, Sarnaik AA, Chen JQ, Creasy C, Kale C, Robinson J, Weber J, Hwu P, Pilon-Thomas S, Radvanyi L (2015) Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res 21(3):611–621. https://doi.org/10.1158/1078-0432.CCR-14-1934
Harao M, Forget MA, Roszik J, Gao H, Babiera GV, Krishnamurthy S, Chacon JA, Li S, Mittendorf EA, DeSnyder SM, Rockwood KF, Bernatchez C, Ueno NT, Radvanyi LG, Vence L, Haymaker C, Reuben JM (2017) 4-1BB-Enhanced Expansion of CD8+ TIL from triple-negative breast cancer unveils mutation-specific CD8+ T cells. Cancer Immunol Res 5(6):439–445. https://doi.org/10.1158/2326-6066.CIR-16-0364
Sakellariou-Thompson D, Forget MA, Creasy C, Bernard V, Zhao L, Kim YU, Hurd MW, Uraoka N, Parra ER, Kang Y, Bristow CA, Rodriguez-Canales J, Fleming JB, Varadhachary G, Javle M, Overman MJ, Alvarez HA, Heffernan TP, Zhang J, Hwu P, Maitra A, Haymaker C, Bernatchez C (2017) 4-1BB agonist focuses CD8(+) Tumor-infiltrating T-Cell growth into a distinct repertoire capable of tumor recognition in pancreatic cancer. Clin Cancer Res 23(23):7263–7275. https://doi.org/10.1158/1078-0432.CCR-17-0831
Tavera RJ, Forget MA, Kim YU, Sakellariou-Thompson D, Creasy CA, Bhatta A, Fulbright OJ, Ramachandran R, Thorsen ST, Flores E, Wahl A, Gonzalez AM, Toth C, Wardell S, Mansaray R, Radvanyi LG, Gombos DS, Patel SP, Hwu P, Amaria RN, Bernatchez C, Haymaker C (2018) Utilizing T-cell activation signals 1, 2, and 3 for tumor-infiltrating lymphocytes (TIL) expansion: the advantage over the sole use of interleukin-2 in cutaneous and uveal melanoma. J Immunother. https://doi.org/10.1097/CJI.0000000000000230
Forget MA, Malu S, Liu H, Toth C, Maiti S, Kale C, Haymaker C, Bernatchez C, Huls H, Wang E, Marincola FM, Hwu P, Cooper LJ, Radvanyi LG (2014) Activation and propagation of tumor-infiltrating lymphocytes on clinical-grade designer artificial antigen-presenting cells for adoptive immunotherapy of melanoma. J Immunother 37(9):448–460. https://doi.org/10.1097/CJI.0000000000000056
Moon BI, Kim TH, Seoh JY (2015) Functional modulation of regulatory T Cells by IL-2. PLoS One 10(11):e0141864. https://doi.org/10.1371/journal.pone.0141864
Haymaker CL, Wu RC, Ritthipichai K, Bernatchez C, Forget MA, Chen JQ, Liu H, Wang E, Marincola F, Hwu P, Radvanyi LG (2015) BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology 4(8):e1014246. https://doi.org/10.1080/2162402X.2015.1014246
Ritthipichai K, Haymaker CL, Martinez M, Aschenbrenner A, Yi X, Zhang M, Kale C, Vence LM, Roszik J, Hailemichael Y, Overwijk WW, Varadarajan N, Nurieva R, Radvanyi LG, Hwu P, Bernatchez C (2017) Multifaceted role of BTLA in the control of CD8(+) T-cell fate after antigen encounter. Clin Cancer Res 23(20):6151–6164. https://doi.org/10.1158/1078-0432.CCR-16-1217
Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N (2007) Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol 178(7):4112–4119. https://doi.org/10.4049/jimmunol.178.7.4112
Shin H, Wherry EJ (2007) CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol 19(4):408–415. https://doi.org/10.1016/j.coi.2007.06.004
Crome SQ, Nguyen LT, Lopez-Verges S, Yang SY, Martin B, Yam JY, Johnson DJ, Nie J, Pniak M, Yen PH, Milea A, Sowamber R, Katz SR, Bernardini MQ, Clarke BA, Shaw PA, Lang PA, Berman HK, Pugh TJ, Lanier LL, Ohashi PS (2017) A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat Med 23(3):368–375. https://doi.org/10.1038/nm.4278
Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH (2014) Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res 20(2):434–444. https://doi.org/10.1158/1078-0432.CCR-13-1877
Komdeur FL, Wouters MC, Workel HH, Tijans AM, Terwindt AL, Brunekreeft KL, Plat A, Klip HG, Eggink FA, Leffers N, Helfrich W, Samplonius DF, Bremer E, Wisman GB, Daemen T, Duiker EW, Hollema H, Nijman HW, de Bruyn M (2016) CD103 + intraepithelial T cells in high-grade serous ovarian cancer are phenotypically diverse TCRalphabeta + CD8alphabeta + T cells that can be targeted for cancer immunotherapy. Oncotarget 7(46):75130–75144. https://doi.org/10.18632/oncotarget.12077
Solberg OD, Mack SJ, Lancaster AK, Single RM, Tsai Y, Sanchez-Mazas A, Thomson G (2008) Balancing selection and heterogeneity across the classical human leukocyte antigen loci: a meta-analytic review of 497 population studies. Hum Immunol 69(7):443–464. https://doi.org/10.1016/j.humimm.2008.05.001
Yokokawa J, Palena C, Arlen P, Hassan R, Ho M, Pastan I, Schlom J, Tsang KY (2005) Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res 11(17):6342–6351. https://doi.org/10.1158/1078-0432.CCR-05-0596
Rahma OE, Ashtar E, Czystowska M, Szajnik ME, Wieckowski E, Bernstein S, Herrin VE, Shams MA, Steinberg SM, Merino M, Gooding W, Visus C, Deleo AB, Wolf JK, Bell JG, Berzofsky JA, Whiteside TL, Khleif SN (2012) A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol Immunother 61(3):373–384. https://doi.org/10.1007/s00262-011-1100-9
Deniger DC, Pasetto A, Robbins PF, Gartner JJ, Prickett TD, Paria BC, Malekzadeh P, Jia L, Yossef R, Langhan MM, Wunderlich JR, Danforth DN, Somerville RPT, Rosenberg SA (2018) T-cell responses to TP53 “hotspot” mutations and unique neoantigens expressed by human ovarian cancers. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-18-0573
Hardwick NR, Frankel P, Ruel C, Kilpatrick J, Tsai W, Kos F, Kaltcheva T, Leong L, Morgan R, Chung V, Tinsley R, Eng M, Wilczynski S, Ellenhorn JDI, Diamond DJ, Cristea M (2018) p53-reactive T cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy. Clin Cancer Res 24(6):1315–1325. https://doi.org/10.1158/1078-0432.CCR-17-2709
Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, Lele S, duPont N, Edwards R, Shrikant P, Old LJ, Gnjatic S, Jager E (2012) Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA 109(15):5797–5802. https://doi.org/10.1073/pnas.1117208109
Lanitis E, Smith JB, Dangaj D, Flingai S, Poussin M, Xu S, Czerniecki BJ, Li YF, Robbins PF, Powell DJ Jr (2014) A human ErbB2-specific T-cell receptor confers potent antitumor effector functions in genetically engineered primary cytotoxic lymphocytes. Hum Gene Ther 25(8):730–739. https://doi.org/10.1089/hum.2014.006
Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P (2006) A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 12(20 Pt 1):6106–6115. https://doi.org/10.1158/1078-0432.CCR-06-1183
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van’t Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome I, Consortium IBC, Consortium IM-S, PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR (2013) Signatures of mutational processes in human cancer. Nature 500(7463):415–421. https://doi.org/10.1038/nature12477
Martin SD, Brown SD, Wick DA, Nielsen JS, Kroeger DR, Twumasi-Boateng K, Holt RA, Nelson BH (2016) Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS One 11(5):e0155189. https://doi.org/10.1371/journal.pone.0155189
Acknowledgements
The authors wish to acknowledge the patients who consented to tissue procurement for research and their caregivers. Thank you to Bristol-Myers Squibb for providing Urelumab and Prometheus for the IL-2. The authors would like to thank René J. Tavera for his early contributions to culturing OvCa TIL.
Funding
This research was supported in part by the MD Anderson Cancer Center Support Grant (P30 CA016672), a T32 training grant for gynecologic oncology (CA101642), the MD Anderson Ovarian Cancer Moon Shot program, and an MD Anderson Institutional Research Grant (Amir A. Jazaeri). We also acknowledge the support of the Flow Cytometry and Cellular Imaging, Research Histology, and Characterized Cell Line cores which are also supported by the support grant (P30 CA016672).
Author information
Authors and Affiliations
Contributions
DST conducted all the experiments and data analysis. DST and MAF generated the TIL lines and prepared the figures. DST, MAF, CH, and CB were responsible for writing the manuscript, while all authors contributed to manuscript editing and approved the final manuscript version. JC oversaw the consent and collection of human samples. MAF, CH, and CB helped with interpretation of the data. JC, EH, and AAJ helped with patients’ clinical information. PH and AAJ provided support and guidance for the study. DST, MAF, CH, and CB designed the experiments and the conceptual design of the study.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the 1975 Helsinki declaration. Ethical approval and tissue from surgical resections used to expand TIL were both obtained under protocols (PA16-0912 and LAB02-188) approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center.
Informed consent
Written informed consent was obtained from all individual participants included in the study for their specimens and data to be used in research and for publication.
Cell line authentication
COV318 and COV362 were originally purchased from Sigma–Aldrich (now Millipore-Sigma, St. Louis, MO) and SKOV3 from the American Type Culture Collection (ATCC, Manassas, VA). All OvCa cell lines were HLA-typed, STR fingerprinted, and confirmed mycoplasma-free at MDACC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sakellariou-Thompson, D., Forget, MA., Hinchcliff, E. et al. Potential clinical application of tumor-infiltrating lymphocyte therapy for ovarian epithelial cancer prior or post-resistance to chemotherapy. Cancer Immunol Immunother 68, 1747–1757 (2019). https://doi.org/10.1007/s00262-019-02402-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02402-z