Abstract
Background
Immune checkpoint inhibitors are now standard-of-care treatments for metastatic cutaneous melanoma. However, for rare sub-groups, such as mucosal melanomas, few published data are available, and with no established therapeutic guidelines. Our objective was to assess the response to anti-CTLA4 and anti-PD1 immunotherapy in patients with mucosal melanomas.
Methods
We performed a single-center, prospective cohort analysis of patients with non-surgical locally advanced and/or metastatic mucosal melanoma receiving anti-CTLA4 and/or anti-PD1 immunotherapy from 2010 to 2016.
Results
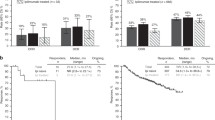
Forty-four patients were enrolled, including 18 (40.9%) with head and neck, 12 (27.3%) with vulvo-vaginal and 14 (31.8%) with ano-rectal primary tumours. Eleven (25%) patients had stage 3 disease, and 11 (25%) had distant metastases. The first-line immunotherapy was ipilimumab in 24 patients and pembrolizumab in 20. The objective response rate (ORR) was 8.2% (one complete response) for ipilimumab and 35% (four complete responses) for pembrolizumab. No significant difference was observed for primary tumour location. The median follow-up was 24 months (range 4–73). The median progression-free survival (PFS) in the first-line ipilimumab and pembrolizumab groups was 3 months [95% confidence interval (CI) 2.5–4.6] and 5 months (95% CI 2.6–33.1), respectively (p = 0.0147).
Conclusion
In the patients with unresectable and/or metastatic mucosal melanoma, we found ORR and PFS rates comparable to those in patients with cutaneous melanoma, with no significant differences in the types of mucosal surfaces involved. Anti-PD1 therapy has a more favorable benefit-risk ratio than ipilimumab and should be used preferentially.


Similar content being viewed by others
Abbreviations
- BRAF:
-
B-Raf proto-oncogene, serine/threonine kinase
- BRCA1:
-
Breast cancer 1
- cKIT:
-
KIT proto-oncogene, receptor tyrosine kinase
- CR:
-
Complete response
- DCR:
-
Disease control rate
- DOR:
-
Duration of response
- iRECIST:
-
Immune response evaluation criteria in solid tumours
- irRC:
-
Immune-related response criteria
- MBD4:
-
Methyl-CpG-binding domain 4
- NF1:
-
Neurofibromin 1
- NRAS:
-
NRAS proto-oncogene, GTPase
- ORR:
-
Objective response rate
- PD:
-
Progressive disease
- SD:
-
Stable disease
- SF3B1:
-
Splicing factor 3b subunit 1
- SNV:
-
Somatic nucleotide variants
- TG:
-
Tumour growth
- TGR:
-
Tumor growth rate
- V0:
-
Tumour volume at baseline
- Vt :
-
Tumour volume at time t
References
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144. https://doi.org/10.1056/NEJMoa1305133
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. https://doi.org/10.1056/NEJMoa1200690
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030. https://doi.org/10.1200/JCO.2013.53.0105
Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R et al (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384:1109–1117. https://doi.org/10.1016/S0140-6736(14)60958-2
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532. https://doi.org/10.1056/NEJMoa1503093
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C et al (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16:908–918. https://doi.org/10.1016/S1470-2045(15)00083-2
Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B et al (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384. https://doi.org/10.1016/S1470-2045(15)70076-8
McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW (2005) Incidence of noncutaneous melanomas in the U.S. Cancer 103:1000–1007. https://doi.org/10.1002/cncr.20866
Patrick RJ, Fenske NA, Messina JL (2007) Primary mucosal melanoma. J Am Acad Dermatol 56:828–834. https://doi.org/10.1016/j.jaad.2006.06.017
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96. https://doi.org/10.3322/CA.2007.0010
Chang AE, Karnell LH, Menck HR (1998) The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83:1664–1678. https://doi.org/10.1002/(sici)1097-0142(19981015)83:8%3c1664:aid-cncr23%3e3.0.co;2-g
Kuk D, Shoushtari AN, Barker CA, Panageas KS, Munhoz RR, Momtaz P et al (2016) Prognosis of mucosal, uveal, acral, non acral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 21:848–854. https://doi.org/10.1634/theoncologist.2015-0522
Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T et al (2017) Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol 79:651–660. https://doi.org/10.1007/s00280-016-3237-x
D’Angelo SP, Larkin J, Sosman JA, Lebbé C, Brady B, Neyns B et al (2017) Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients With mucosal melanoma: a pooled analysis. J Clin Oncol 35:226–235. https://doi.org/10.1200/JCO.2016.67.9258
Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK et al (2016) The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 122:3354–3362. https://doi.org/10.1002/cncr.30259
Del Vecchio M, Di Guardo L, Ascierto PA, Grimaldi AM, Sileni VC, Pigozzo J et al (2014) Efficacy and safety of ipilimumab 3 mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer 50:121–127. https://doi.org/10.1016/j.ejca.2013.09.007
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S et al (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920–1928. https://doi.org/10.1158/1078-0432.CCR-16-1741
Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM et al (2018) Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol 36:1668–1674. https://doi.org/10.1200/JCO.2017.75.6270
Wolchock JD, Chiaron-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356
Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF et al (2016) Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17:1558–1568. https://doi.org/10.1016/S1470-2045(16)30366-7
Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R (2017) Ipilimumab alone or in combination with nivolumab after progression on anti-PD1 therapy in advanced melanoma. Eur J Cancer 75:47–55
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A et al (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371:2189–2199. https://doi.org/10.1056/NEJMoa1406498
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV et al (2013) Signatures of mutational processes in human cancer. Nature 500:415–421. https://doi.org/10.1038/nature12477
Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB et al (2018) High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553:347–350. https://doi.org/10.1038/nature25187
Furney SJ, Turajlic S, Stamp G, Nohadani M, Carlisle A, Thomas JM et al (2013) Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol 230:261–269. https://doi.org/10.1002/path.4204
Furney SJ, Turajlic S, Stamp G, Thomas JM, Hayes A, Strauss D et al (2014) The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell Melanoma Res 27:835–838. https://doi.org/10.1111/pcmr.12279
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K et al (2017) Whole-genome landscapes of major melanoma subtypes. Nature 545:175–180. https://doi.org/10.1038/nature22071
Hintzsche JD, Gorden NT, Amato CM, Kim J, Wuensch KE, Robinson SE et al (2017) Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res 27:189–199. https://doi.org/10.1097/CMR.0000000000000345
Van Engen-van Grunsven AC, Baar MP, Pfundt R, Rijntjes J, Küsters-Vandevelde HV, Delbecq AL et al (2015) Whole-genome copy-number analysis identifies new leads for chromosomal aberrations involved in the oncogenesis and metastastic behavior of uveal melanomas. Melanoma Res 25:200–209. https://doi.org/10.1097/CMR.0000000000000152
Furney SJ, Pedersen M, Gentien D, Dumont AG, Rapinat A, Desjardins L et al (2013) SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov 2013(3):1122–1129. https://doi.org/10.1158/2159-8290.CD-13-0330
Heppt MV, Heinzerling L, Kähler KC, Forschner A, Kirchberger MC, Loquai C et al (2017) Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer 82:56–65. https://doi.org/10.1016/j.ejca.2017.05.038
Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM et al (2016) Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 122:3344–3353. https://doi.org/10.1002/cncr.30258
Rodrigues M, Mobuchon L, Houy A, Fiévet A, Gardrat S, Barnhill RL et al (2018) Outlier response to anti-pd1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat Commun. 9(1):1866. https://doi.org/10.1038/s41467-018-04322-5
Funding
No relevant funding.
Author information
Authors and Affiliations
Contributions
AM-P, FJ, AMME, LD and CR conceptualized the manuscript, harmonized and edited the text and references and produced the figures. CR and IG performed statistical analysis. RGHG, SA, SR, J-YS and SV were responsible for data collection and analysis. All the authors contributed to the writing and editing of the manuscript. All the authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The translational research study NCT02105168 was approved by the Institutional Review Board at Gustave Roussy Cancer Campus on April 7, 2014. All the methods and procedures associated with this study were conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All the patients have given their written authorization to perform research on their tumour samples by signing the Institutional Tumor Bank form (before April 2014) or the form of the translational research study NCT02105168. All the patients provided written informed consent to the use of their anonymized data in scientific studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Moya-Plana A, Herrera Gómez RG, Rossoni C, Dercle L, Ammari S, Girault I et al. “Response assessment to anti-CTLA4 or/and anti-PD1 immunotherapy in mucosal melanomas”. American Society of Clinical Oncology (ASCO) Annual Meeting, June 2018 Chicago, USA. [Abstract #221719].
Rights and permissions
About this article
Cite this article
Moya-Plana, A., Herrera Gómez, R.G., Rossoni, C. et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother 68, 1171–1178 (2019). https://doi.org/10.1007/s00262-019-02351-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02351-7