Abstract
Background
Mammary and extra-mammary Paget disease is a rare form of intra-epithelial glandular neoplasm which is characteristically recurrent and necessitates multiple excisions that have an important impact on morbidity. Local immuno-modulating treatments have been applied with promising results, but the local immune markers of Paget disease have not been studied.
Aim of the study
To investigate the local immune micro-environment of Paget disease.
Materials and methods
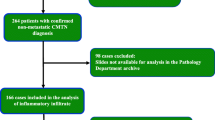
Sixty-four specimens from 41 patients, including cases with multiple recurrences and underlying primary neoplasm, have been studied for their expression of CD3, PD-L1 and CTLA-4.
Results
Nineteen cases were mammary; 22 were extra-mammary and involved the vulva, the anus, the inguinal region and the lower extremity. PD-L1 was not expressed by any neoplastic lesion or the associated lymphocytes. CTLA-4 expression was found in nine cases. Higher stromal CD3 expression and moderate levels of intra-epithelial CD3 expression were present in most cases. Biopsies, subsequent excision specimens and recurrences showed the same immunohistochemical profile of CD3 and PD-L1, although there were different levels of CTLA-4 in a few cases. The underlying lesions in mammary Paget disease showed the same immunohistochemical profile as the intra-epithelial neoplastic cells. The expression of the markers did not correlate with age, sex, localization or recurrence.
Conclusion
Paget disease is characterized by an intense lymphocytic response, devoid of the immune-suppressive impact of the PD-L1 pathway, but with occasional CTLA-4 expression.


Similar content being viewed by others
Abbreviations
- CIN:
-
Cervical intra-epithelial neoplasia
- DCIS:
-
Ductal carcinoma in situ
- EMPD:
-
Extra-mammary Paget disease
- FDA:
-
Food and Drug Administration
- MPD:
-
Mammary Paget disease
- PD:
-
Paget disease
- RANK/RANKL:
-
Receptor activator of nuclear factor kappa B ligand
References
Lloyd J, Flanagan AM (2000) Mammary and extramammary Paget’s disease. J Clin Pathol 53(10):742–749
Duan X, Sneige N, Gullett AE, Prieto VG, Resetkova E, Andino LM et al (2012) Invasive paget disease of the breast: clinicopathologic study of an underrecognized entity in the breast. Am J Surg Pathol 36(9):1353–1358
Lam C, Funaro D (2010) Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin 28(4):807–826
Kazakov DV, Spagnolo DV, Kacerovska D, Michal M (2011) Lesions of anogenital mammary-like glands. Adv Anat Pathol 18(1):1–28
Konstantinova AM, Shelekhova KV, Stewart CJ, Spagnolo DV, Kutzner H, Kacerovska D et al (2016) Depth and patterns of adnexal involvement in primary extramammary (anogenital) Paget disease: a study of 178 lesions from 146 patients. Am J Dermatopathol 38(11):802–808
Edey KA, Allan E, Murdoch JB, Cooper S, Bryant A (2013) Interventions for the treatment of Paget’s disease of the vulva. Cochrane Database Syst Rev (Wiley, Chichester)
Cowan RA, Black DR, Hoang LN, Park KJ, Soslow RA, Backes FJ et al (2016) A pilot study of topical imiquimod therapy for the treatment of recurrent extramammary Paget’s disease. Gynecol Oncol 142(1):139–143
Fletcher J, Haniffa M (2017) Mechanisms of immune evasion in extramammary Paget disease. Br J Dermatol 176(2):293–294
Fujimura T, Kambayashi Y, Furudate S, Kakizaki A, Hidaka T, Aiba S (2017) Possible mechanisms of the crosstalk between Langerhans cells and regulatory T cells in extramammary Paget disease by receptor activator of nuclear factor kappa B (RANK) ligand/RANK pathways. Br J Dermatol 176(2):387–394
Karpathiou G, Casteillo F, Giroult JB, Forest F, Fournel P, Monaya A et al (2017) Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget 8(12):19310–19322
Padrnos L, Karlin N, Halfdanarson TR (2016) Mayo Clinic Cancer Center experience of metastatic extramammary Paget disease 1998–2012. Rare Tumors 8(4):6804
Onaiwu CO, Salcedo MP, Pessini SA, Munsell MF, Euscher EE, Reed KE et al (2017) Paget’s disease of the vulva: a review of 89 cases. Gynecol Oncol Rep 19:46–49
Jones ISC, Crandon A, Sanday K (2011) Paget’s disease of the vulva: diagnosis and follow-up key to management; a retrospective study of 50 cases from Queensland. Gynecol Oncol 122(1):42–44
Budhu S, Wolchok J, Merghoub T (2014) The importance of animal models in tumor immunity and immunotherapy. Curr Opin Genet Dev 24:46–51
Press JZ, Allison KH, Garcia R, Everett EN, Pizer E, Swensen RE et al (2011) FOXP3+ regulatory T-cells are abundant in vulvar Paget’s disease and are associated with recurrence. Gynecol Oncol 120(2):296–299
Sznurkowski JJ, Żawrocki A, Biernat W (2017) Local immune response depends on p16INK4a status of primary tumor in vulvar squamous cell carcinoma. Oncotarget 8(28):46204–46210
Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F et al (2017) Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov 7(10):1099–1115
Hendry S, Pang JMB, Byrne DJ, Lakhani SR, Cummings MC, Campbell IG et al (2017) Relationship of the breast ductal carcinoma in situ immune microenvironment with clinicopathological and genetic features. Clin Cancer Res 23(17):5210–5217
Thompson E, Taube JM, Elwood H, Sharma R, Meeker A, Warzecha HN et al (2016) The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol 29(3):249–258
Yang W, Song Y, Lu Y-L, Sun J-Z, Wang H-W (2013) Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology 139(4):513–522
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ et al (2015) Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230):124–128
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm560167.htm
Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L et al (2016) PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 374(26):2542–2552
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Acknowledgements
The authors would like to thank Mr Philippe Cosmo from the Tumorothèque/Centre de Ressources Biologiques de CHU Saint-Etienne (BRIF no. BB-0033-00041) for his assistance.
Funding
No relevant funding.
Author information
Authors and Affiliations
Contributions
GK conceived the study. GK and MP designed the study. GK, CC, SH and CH reviewed the clinical files. GK and SH executed the laboratory techniques. All authors were involved in data analysis and interpretation. GK wrote the manuscript. CC, SH, CH and MP critically revised the manuscript. All authors approved the final form.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Local Ethics Committee of the University Hospital of Saint-Etienne approved the study (IRBN132018/CHUSTE).
Informed consent
The acquisition of written informed consent was waived by the institutional review board given the retrospective nature of the study and the anonymization of all data.
Rights and permissions
About this article
Cite this article
Karpathiou, G., Chauleur, C., Hathroubi, S. et al. Expression of CD3, PD-L1 and CTLA-4 in mammary and extra-mammary Paget disease. Cancer Immunol Immunother 67, 1297–1303 (2018). https://doi.org/10.1007/s00262-018-2189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2189-x