Abstract
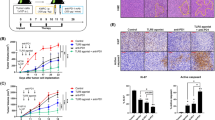
High-mobility group box 1 (HMGB1) is involved in the tumor-associated activation of regulatory T cells (Treg), but the mechanisms remain unknown. In a mouse tumor model, silencing HMGB1 in tumor cells or inhibiting tumor-derived HMGB1 not only dampened the capacity of tumor cells to produce thymic stromal lymphopoietin (TSLP), but also aborted the tumor-associated modulation of Treg-activating DC. Tumor-derived HMGB1 triggered the production of TSLP by tumor cells. Importantly, both tumor-derived HMGB1 and TSLP were necessary for modulating DC to activate Treg in a TSLP receptor (TSLPR)-dependent manner. In the therapeutic model, intratumorally inhibiting tumor-derived HMGB1 (causing downstream loss of TSLP production) attenuated Treg activation, unleashed tumor-specific CD8 T cell responses, and elicited CD8α+/CD103+DC- and T cell-dependent antitumor activity. These results suggest a new pathway for the activation of Treg involving in tumor-derived HMGB1 and TSLP, and have important implications for incorporating HMGB1 inhibitors into cancer immunotherapy.





Similar content being viewed by others
Abbreviations
- APC:
-
Antigen-presenting cells
- Box A:
-
An antagonist for HMGB1
- EP:
-
Ethyl pyruvate
- GL:
-
Glycyrrhizin
- HMGB1:
-
High-mobility group box 1
- I.t.:
-
Intratumorally
- KD:
-
Knockdown
- KI:
-
Knockin
- shRNA:
-
Short hairpin RNA
- SPC:
-
Splenocytes
- TDLN:
-
Tumor-draining lymph nodes
- Th1 CD4+ :
-
T helper 1
- Th2 CD4+ :
-
T helper 2
- TME:
-
Tumor microenvironment
- Treg Foxp3+ :
-
T regulatory cells
- TSLP:
-
Thymic stromal lymphopoietin
- TSLPR:
-
TSLP receptor
- WB:
-
Western blot
- WT:
-
Wild type
References
Zou W (2006) Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol 6:295–307
Beyer M, Schultze JL (2009) Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des 15:1879–1892
Savage PA (2013) Basic principles of tumor-associated regulatory T cell biology. Trends Immunol 34:33–40
Takeuchi Y, Nishikawa H (2016) Roles of regulatory T cells in cancer immunity. Int Immunol 28:401–409
Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT (2007) Masquerader: high mobility group box-1 and cancer. Clin Cancer Res 13:2836–2848
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, André F, Delaloge S, Tursz T, Kroemer G, Zitvogel L (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059
Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG (2009) HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 6:e10
Guerriero JL, Ditsworth D, Catanzaro JM, Sabino G, Furie MB, Kew RR, Crawford HC, Zong WX (2011) DNA alkylating therapy induces tumor regression through an HMGB1-mediated activation of innate immunity. J Immunol 186:3517–3526
Gdynia G, Sauer SW, Kopitz J, Fuchs D, Duglova K, Ruppert T, Miller M, Pahl J, Cerwenka A, Enders M, Mairbäurl H, Kamiński MM, Penzel R, Zhang C, Fuller JC, Wade RC, Benner A, Chang-Claude J, Brenner H, Hoffmeister M, Zentgraf H, Schirmacher P, Roth W (2016) The HMGB1 protein induces a metabolic type of tumour cell death by blocking aerobic respiration. Nat Commun 7:10764. https://doi.org/10.1038/ncomms10764
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM (2000) Blockade of RAGE-amphoterin signaling suppresses tumour growth and metastases. Nature 405:354–360
Liang X, Chavez AR, Schapiro NE, Loughran P, Thorne SH, Amoscato AA, Zeh HJ, Beer-Stolz D, Lotze MT, de Vera ME (2009) Ethyl pyruvate administration inhibits hepatic tumor growth. J Leukoc Biol 86:599–607
Campana L, Bosurgi L, Rovere-Querini P (2008) HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol 20:518–523
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ (2010) HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367–388
Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, Pass HI, Gaudino G, Carbone M, Yang H (2012) Cancer Cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res 72:3290–3301
Kusume A, Sasahira T, Luo Y, Isobe M, Nakagawa N, Tatsumoto N, Fujii K, Ohmori H, Kuniyasu H (2009) Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology 76:155–162
Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M (2012) Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol 13:832–842
Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, Tracey KJ, Ostrand-Rosenberg S (2014) HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res 74:5723–5733
Su Z, Ni P, She P, Liu Y, Richard SA, Xu W, Zhu H, Wang J (2016) Bio-HMGB1 from breast cancer contributes to M-MDSC differentiation from bone marrow progenitor cells and facilitates conversion of monocytes into MDSC-like cells. Cancer Immunol Immunother 66:391–401. https://doi.org/10.1007/s00262-016-1942-2
Liu Z, Falo LD Jr, You Z (2011) Knockdown of HMGB1 in tumor cells attenuates their ability to induce regulatory T cells and uncovers naturally acquired CD8 T cell-dependent antitumor immunity. J Immunol 187:118–125
Wild CA, Brandau S, Lotfi R, Mattheis S, Gu X, Lang S, Bergmann C (2012) HMGB1 is overexpressed in tumor cells and promotes activity of regulatory T cells in patients with head and neck cancer. Oral Oncol 48:409–416
Wild CA, Bergmann C, Fritz G, Schuler P, Hoffmann TK, Lotfi R, Westendorf A, Brandau S, Lang S (2012) HMGB1 conveys immunosuppressive characteristics on regulatory and conventional T cells. Int Immunol 24:485–494
Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, Tanaka Y, Zurawski S, Zurawski G, Bover L, Liu YJ, Banchereau J, Palucka AK (2011) Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med 208:479–490
Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ, Biragyn A (2011) Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol 186:5656–5662
De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP (2011) Intratumor T helper 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 208:469–478
Erdmann RB, Gartner JG, Leonard WJ, Ellison CA (2013) Lack of functional TSLP receptors mitigates Th2 polarization and the establishment and growth of 4T1 primary breast tumours but has different effects on tumour quantities in the lung and brain. Scand J Immunol 78:408–418
Lo Kuan E, Ziegler SF (2014) Thymic stromal lymphopoietin and cancer. J Immunol 193:4283–4288
Barooei R, Mahmoudian RA, Abbaszadegan MR, Mansouri A, Gholamin M (2015) Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Med Oncol 32:217
De Jesus-Carrion S, Ziegler SF (2016) Control of tumor growth by TSLP during colorectal cancer. J Immunol 196:73.3 (Abstract from IMMUNOLOGY 2016™, May 13–17, 2016, Seattle, USA)
Ziegler SF, Artis D (2010) Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol 11:289–293. https://doi.org/10.1038/ni.1852
Redhu NS, Gounni AS (2012) Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin Exp Allergy 42:994–1005
Wu TC, Xu K, Banchereau J, Palucka AK (2016) Cancer cell-derived transforming growth factor (TGF)-β and myeloid cell-derived interleukin (IL)-1β synergize to promote type 2 inflammation and breast cancer progression. J Immunol 196:73.8 (Abstract from IMMUNOLOGY 2016™, May 13–17, 2016, Seattle, USA)
Zhang Y, Tian S, Liu Z, Zhang J, Zhang M, Bosenberg MW, Kedl RM, Waldmann TA, Storkus WJ, Falo LD Jr, You Z (2014) Dendritic cell-derived interleukin-15 is crucial for therapeutic cancer vaccine potency. OncoImmunology 3:e959321
Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF (2005) Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6:1047–1053
He Y, Zhang J, Mi Z, Robbins P, Falo LD Jr (2005) Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol 174:3808–3817
Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA (2008) Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29:21–32
Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME (2007) Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 14:431–441
Liu Z, Kim JH, Falo LD Jr, You Z (2009) Tumor regulatory T cells potently abrogate antitumor immunity. J Immunol 182:6160–6167
Musumeci D, Roviello GN, Montesarchio D (2014) An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther 141:347–357
Yang H, Oceani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaima R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton M, Anderson U, Tracey KJ (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 101:296–301
Tang D, Kang R, Zeh HJ 3rd, Lotze MT (2010) High-mobility group box 1 and cancer. Biochim Biophys Acta 1799:131–140
Rojas A, Figueroa H, Morales E (2010) Fueling inflammation at tumor microenvironment: the role of multiligand/rage axis. Carcinogenesis 31:334–341
He Y, Zha J, Wang Y, Liu W, Yang X, Yu P (2013) Tissue damage-associated “danger signals” influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Res 73:629–639
Cottone L, Capobianco A, Gualteroni C, Monno A, Raccagni I, Valtorta S, Canu T, Di Tomaso T, Lombardo A, Esposito A, Moresco RM, Maschio AD, Naldini L, Rovere-Querini P, Bianchi ME, Manfredi AA (2016) Leukocytes recruited by tumor-derived HMGB1 sustain peritoneal carcinomatosis. Oncoimmunology 5:e1122860
Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5:825–830
Yang H, Wang H, Czura CJ, Tracey KJ (2005) The cytokine activity of HMGB1. J Leukoc Biol 78:1–8
Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P (2005) HMGB1: guiding immunity from within. Trends Immunol 26:381–387
Bianchi ME, Manfredi AA (2007) High mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 220:35–46
Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ (2007) Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496
Sha Y, Zmijewski J, Xu Z, Abraham E (2008) HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 180:2531–2537
Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT, Tang D (2013) HMGB1 in cancer: good, bad, or both. Clin Cancer Res 19:4046–4057
Tsan MF (2011) Heat shock proteins and high mobility group box 1 protein lack cytokine function. J Leukoc Biol 89:847–853
Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209:551–563
Hreggvidsdóttir HS, Lundberg AM, Aveberger AC, Klevenvall L, Andersson U, Harris HE (2012) High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol Med 18:224–230
Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME (2012) Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 209:1519–1528
Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F (2012) Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell 22:479–493
Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R (2012) Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 22:494–505
Demehri S, Cunningham TJ, Manivasagam S, Ngo KH, Moradi Tuchayi S, Reddy R, Meyers MA, DeNardo DG, Yokoyama WM (2016) Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J Clin Invest 126:1458–1470
Lo Kuan E, Ziegler SF (2016) Thymic stromal lymphopoietinpromotes interplay between breast tumor cells, neutrophils and Ly6Chi monocytes to regulate breast tumor progression. J Immunol 196:72 (Abstract from IMMUNOLOGY 2016™, May 13–17, 2016, Seattle, USA)
Lee HC, Ziegler SF (2007) Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NF-κB. Proc Natl Acad Sci USA 104:914–919
Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ (2010) A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942–11947
Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ (2005) Hassall’s corpuscles instruct dendritic cells to induce CD4 + CD25 + regulatory T cells in human thymus. Nature 436:1181–1185
Suzuki F, Schmitt DA, Utsunomiya T, Pollard RB (1992) Stimulation of host resistance against tumors by glycyrrhizin, an active component of licorice roots. In Vivo 6:589–596
Hsiang CY, Lai IL, Chao DC, Ho TY (2002) Differential regulation of activator protein 1 activity by glycyrrhizin. Life Sci 70:1643–1656
Kobayashi M, Fujita K, Katakura T, Utsunomiya T, Pollard RB, Suzuki F (2002) Inhibitory effect of glycyrrhizin on experimental pulmonary metastasis in mice inoculated with B16 melanoma. Anticancer Res 22:4053–4058
Hibasami H, Iwase H, Yoshioka K, Takahashi H (2005) Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int J Mol Med 16:233–236
Rossi T, Benassi L, Magnoni C, Ruberto AI, Coppi A, Baggio G (2005) Effects of glycyrrhizin on UVB-irradiated melanoma cells. In Vivo 19:319–322
Niwa K, Lian Z, Onogi K, Yun W, Tang L, Mori H, Tamaya T (2007) Preventive effects of glycyrrhizin on estrogen-related endometrial carcinogenesis in mice. Oncol Rep 17:617–622
Thirugnanam S, Xu L, Ramaswamy K, Gnanasekar M (2008) Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol Rep 20:1387–1392
Raphael TJ, Kuttan G (2008) Effect of naturally occurring triterpenoids ursolic acid and glycyrrhizic acid on the cell-mediated immune responses of metastatic tumor-bearing animals. Immunopharmacol Immunotoxicol 30:243–255
Sharma G, Kar S, Palit S, Das PK (2012) 18β-Glycyrrhetinic acid induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J Cell Physiol 227:1923–1931
Acknowledgements
We are indebted to Steven F. Ziegler (Benaroya Research Institute) for providing critical experimental materials which were prepared by Whitney Xu at the laboratory of Steven F. Ziegler, Dewayne Falkner at Flow Cytometry Core Facility (University of Pittsburgh) for assisting in flow cytometry and cell sorting, and Stephen C. Balmert at the laboratory of Louis D. Falo, Jr. for editing the manuscript. This work was supported by Department of Dermatology at The University of Pittsburgh School of Medicine and NIH Grants R21CA191522 (Zhaoyang You) and P50CA121973 (Louis D. Falo, Jr.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, Z., Hao, X. et al. Tumor-derived high-mobility group box 1 and thymic stromal lymphopoietin are involved in modulating dendritic cells to activate T regulatory cells in a mouse model. Cancer Immunol Immunother 67, 353–366 (2018). https://doi.org/10.1007/s00262-017-2087-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2087-7