Abstract
Regulatory T cells (Tregs) have been proposed to dampen functions of anti-neoplastic immune cells and thus promote cancer progression. In a phase IV trial (Re:Mission Trial, NCT01347996, http://www.clinicaltrials.gov) 84 patients (age 18–79) with acute myeloid leukemia (AML) in first complete remission (CR) received ten consecutive 3-week cycles of immunotherapy with histamine dihydrochloride (HDC) and low-dose interleukin-2 (IL-2) to prevent relapse of leukemia in the post-consolidation phase. This study aimed at defining the features, function and dynamics of Foxp3+CD25highCD4+ Tregs during immunotherapy and to determine the potential impact of Tregs on relapse risk and survival. We observed a pronounced increase in Treg counts in peripheral blood during initial cycles of HDC/IL-2. The accumulating Tregs resembled thymic-derived natural Tregs (nTregs), showed augmented expression of CTLA-4 and suppressed the cell cycle proliferation of conventional T cells ex vivo. Relapse of AML was not prognosticated by Treg counts at onset of treatment or after the first cycle of immunotherapy. However, the magnitude of Treg induction was diminished in subsequent treatment cycles. Exploratory analyses implied that a reduced expansion of Tregs in later treatment cycles and a short Treg telomere length were significantly associated with a favorable clinical outcome. Our results suggest that immunotherapy with HDC/IL-2 in AML entails induction of immunosuppressive Tregs that may be targeted for improved anti-leukemic efficiency.
Similar content being viewed by others
Introduction
Regulatory T cells (Tregs) are Foxp3+CD25highCD4+ T cells with diverse immunosuppressive functions. Subsets of Tregs include natural Tregs (nTregs), that are thymus-derived but undergo further expansion in peripheral tissues, and induced Tregs (iTregs) that are converted from conventional T cells (Tcons) in the periphery [1,2,3]. Both subsets have been shown to suppress autoreactive lymphocytes and thus to limit the magnitude of innate and adaptive immune responses [2, 4, 5]. Accordingly, impaired Treg function aggravates autoimmune diseases while Treg-mediated immunosuppression may inhibit pathogen clearance and promote chronic infection [6, 7]. In addition to controlling autoimmunity, Tregs have been ascribed a role as mediators of cancer-related immunosuppression. Studies in murine models show that Tregs accumulate in several forms of experimental cancer and that depletion of Tregs or strategies to target their immunosuppressive features reduce cancer growth [8, 9]. In many solid human cancers, Tregs accumulate in the tumor microenvironment and their presence typically, albeit not invariably, heralds advanced disease and poor survival [10,11,12,13].
Acute myeloid leukemia (AML) is characterized by rapid expansion of immature myeloid cells in bone marrow and other organs [14]. In AML, the malignant clone is reportedly controlled by cellular immunity, including natural killer (NK) cells and subsets of cytotoxic (CD8+) T cells [15]. While relatively little is known about the role of Tregs for the efficiency of anti-leukemic immunity in AML [16, 17], several other immunosuppressive pathways of relevance to the course of disease have been described [18,19,20] including immunosuppression exerted by NOX2-derived reactive oxygen species (ROS) released from myeloid cells [21]. Under conditions of NOX2-related oxidative stress, targeting of ROS formation using the NOX2 inhibitor histamine dihydrochloride (HDC) upholds NK cell and T cell function and improves the efficiency of NK- and T cell-activating agents such as interleukin-2 (IL-2) [22,23,24,25]. Monotherapy with IL-2 has yielded disappointment in several clinical trials in AML [26,27,28,29,30,31]. However, phase III trial results showed that the combination of HDC and low-dose IL-2 improves the leukemia-free survival (LFS) of AML patients in complete remission (CR) after chemotherapy [32], thus supporting the clinical relevance of NOX2-mediated immunosuppression in AML.
The IL-2 component of the HDC/IL-2 regimen may expand Tregs as these cells express high-affinity IL-2 receptors (CD25) and rely on exogenous IL-2 for proliferation [33, 34]. Treatment with IL-2 has been shown to increase the population of Tregs and reduce graft-versus-host manifestations in cancer patients receiving allogeneic stem cell transplants (allo-SCT) [35,36,37,38]. It is thus conceivable that IL-2-driven Treg expansion may limit the anti-leukemic efficiency of HDC/IL-2 immunotherapy. For the present study, we monitored Treg number and function in AML patients in first CR who received HDC/IL-2 for relapse prevention in a phase IV trial. Our results imply that treatment with HDC/IL-2 entails pronounced accumulation of nTregs in blood and that aspects of Treg function are relevant to relapse risk in AML.
Patients, materials and methods
Patients, study design and objectives
The Re:Mission trial (NCT01347996, registered at http://www.clinicaltrials.gov) was a single-armed multicenter phase IV study that enrolled 84 patients (age 18–79) with confirmed AML in first CR who were not eligible for allo-SCT. The patients received ten consecutive 21-day cycles of HDC/IL-2 during 18 months or until relapse or death. Each cycle comprised 0.5 mg histamine dihydrochloride (HDC; Ceplene®) and 16,400 U/kg human recombinant IL-2 (aldesleukin) that were administered by subcutaneous injection twice daily. The off-treatment periods in cycle 1–3 were 3 weeks, while the off-treatment periods between cycle 4–10 were extended to 6 weeks. All patients were followed for at least 24 months after enrollment. Fourteen patients discontinued prematurely from the study and were censored at the last captured follow-up date. The exclusion criteria for enrollment were identical to those employed in a previous phase III trial [32]. The primary endpoints comprised assessment of the quantitative and qualitative pharmacodynamic properties of HDC/IL-2, including monitoring of T and NK cell phenotypes before and after treatment cycles while analyses of aspects of immunity versus outcome (LFS and overall survival; OS) were performed post hoc. Patient characteristics, including details regarding previous induction and consolidation therapy and risk group distribution are accounted for in previous publications [39,40,41] and in Table 1. The trial was approved by the Ethical Committees of each participating institution and all patients gave written informed consent before enrollment.
Isolation of PBMCs from healthy donors and patient samples
Buffy coats from healthy donors were obtained from the Blood Center at the Sahlgrenska University Hospital, Gothenburg, Sweden. To remove erythrocytes, the blood was mixed at a 1:1 ratio with 2% dextran and left to sediment. The upper phase was transferred to tubes containing Ficoll/Lymphoprep (Alere AB, Lidingö, Sweden) and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation. The PBMCs were cryopreserved until further use. Peripheral blood was collected from patients in the Re:Mission trial before and after the first and third treatment cycles, i.e., cycle 1, day 1 (C1D1) and cycle 1, day 21 (C1D21), cycle 3, day 1 (C3D1) and cycle 3, day 21 (C3D21). Patient PBMCs were isolated and cryopreserved at local sites and shipped on dry ice to the central laboratory (the TIMM Laboratory, Sahlgrenska Cancer Center, University of Gothenburg, Sweden) for analysis.
Staining and flow cytometry
Cryopreserved samples were quickly thawed, washed and stained with LIVE/DEAD fixable yellow stain (Life technologies, Grand Island, NY, USA). Thereafter, cells were washed and incubated with an antibody cocktail for surface markers in PBS containing 0.5% BSA and 0.1% EDTA or in Brilliant stain buffer (BD Biosciences, Stockholm, Sweden). The following anti-human monoclonal antibodies were purchased from BD Biosciences: CD3-FITC (HIT3a), CD3-Brilliant Violet 711 (UCHT1), CD4-APC-H7 (RPA-T4), CD8-PerCP-Cy5.5 (SK1), CD14-FITC (MϕP9), CD25-Brilliant Violet 421 (M-A251), CD56-PE-Cy7 (NCAM16.2) and CD127-AF647 (HIL-7R-M21). CTLA-4-PE-Cy7 (L3D10) was obtained from Biolegend (San Diego, CA, USA) and CD14-Qdot655 (TüK4) from Life Technologies. For intracellular staining with Foxp3-PE (3G3; Miltenyi Biotec, Auburn, CA) and Helios-AF647 (22F6; BD Biosciences), cells were fixed and permeabilized using the Foxp3 fixation/permeabilization kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s protocol. A 4-laser BD LSRFortessa SORP flow cytometer (405, 488, 532, and 640 nm; BD Biosciences) was employed to analyze samples. Data analysis was performed using the FlowJo software, version 7.6.5 or later (TreeStar, Ashland, OR, USA), or FACSDiva software, version 6 or later (BD Biosciences). Samples with less than 25% viability were excluded.
Blood samples were available from 81 out of 84 patients. Differential counts of whole blood were performed at local sites and were utilized to calculate absolute counts of Tregs in blood. Notably, the definition of Tregs in this study was restricted to Foxp3+CD25highCD4+ cells. All available samples were analyzed for Treg content. If an analysis failed according to pre-defined criteria (experimental failure, few cells, poor cellular viability), a second sample was thawed for re-analysis. If the second attempt also failed to generate data, the sample was excluded from analysis. A thorough analysis of expression of Treg markers (including CTLA-4 and Helios) was performed in 25 randomly selected patients. These patients were largely representative of all participating patients in terms of age (mean age for selected group 57.7 years (23.8–76.5 years) vs. mean age for all patients 58.6 years (19–77 years), risk group classification according to recommendations by the European LeukemiaNet [42] [among the selected patients 6 (24%) belonged to the favorable group, 14 (56%) to the intermediate group and 3 (12%) to the adverse group, 2 (8%) not done, whereas among all patients 34 (40.5%) belonged to the favorable group, 38 (45.2%) to the intermediate group and 7 (8.3%) to the adverse group, 5 (6%) not done] and French American British (FAB) classification (data not shown). All successfully analyzed samples, according to the pre-defined criteria stated above, were included in this report.
Treg methylation analysis
Tregs (CD4+CD14−CD25hiCD127low) were sorted from blood samples recovered at the end of treatment cycle 3 (C3D21) and from healthy subjects. Sorted cells (at least 40,000 cells per assay) were washed before being frozen in 200 µl PBS. The DNA methylation status of 15 CpG-motifs within the Treg-specific demethylated region (TSDR) was analyzed by bisulphite sequencing performed by Epiontis GmbH (Berlin, Germany) as previously described [43]. Only male subjects were included in analyses of Treg methylation status cells since the FOXP3 gene locus is located on the X-chromosome [44] and X-chromosome inactivation in females would likely influence results.
Treg suppression assay
Patient samples collected on C3D21 with a Treg content of 15–40% of the CD4+ population were used in Treg suppression assays ex vivo. PBMCs collected from healthy donors served as control. Cells were stained with anti-human monoclonal antibodies as described above. Tregs (CD4+CD14−CD25hiCD127low) and conventional CD4+ T cells (Tcons; CD4+CD14−CD25lowCD127hi) were sorted on a 3-laser BD FACSAriaIII flow cytometer (405, 488 and 640 nm; BD Biosciences). The gating strategy is shown in Supplementary Fig. 2. The sorted Tcons were stained with CellTrace™ violet (Life Technologies) and 35,000 cells per well were seeded together with 2 µg/ml soluble anti-CD28, in X-VIVO™ 15 serum-free medium (Lonza Group Ltd, Basel, Switzerland) to a 384-well plate coated with anti-CD3 (OKT3; eBioscience). An equal number of Tregs (35,000/well) was added to half of the wells. After 4–5 days of culture the proliferation of Tcons was determined by measuring the intensity of the CellTrace™ violet staining on an LSRFortessa SORP flow cytometer (BD Biosciences).
Quantitative PCR telomere length assay
Tregs (CD4+CD25hiCD127low) were sorted from patient blood samples recovered at C3D1 and C3D21 or from healthy controls. Cells were sorted into 96-well plates (Life Technologies) for direct cell lysis and kept at −80 °C until analysis. Optimally, four technical replicates of 400 cells/well were obtained from all blood samples. Protease from Streptomyces griseus (2 μg; Sigma-Aldrich) diluted in PBS (Life Technologies) was added to each well followed by incubation at 37 °C for 10 min and enzyme inactivation at 95 °C for 15 min. The plates were centrifuged at 3000 rpm for 5 min. Quantitative PCR (qPCR) was performed using a CFX384 Touch Real-Time PCR Detection System (Bio-Rad). Primers designed by Cawthon [45] were used for amplification of a short fixed-length product at a copy number proportional to telomere length, and of the single copy gene albumin, in separate wells. Each 10-µl qPCR reaction contained 1X TATAA SYBR GrandMaster Mix (TATAA Biocenter), 400 nM of each primer, and 2 µl protease-treated DNA. Each technical replicate was assayed in duplicate. The thermal cycling profile was 95 °C for 1 min, 2 cycles of 94 °C for 15 s and 49 °C for 15 s, and 40 cycles of amplification (94 °C for 15 s, 62 °C for 10 s and 74 °C for 15 s). Formation of the correct PCR products was confirmed by melting-curve analysis. Relative telomere lengths were determined by normalizing the telomere qPCR signals against signals observed in the corresponding albumin gene assays.
Statistical analyses
Single comparisons of Treg, Tcon and NK cell phenotypes were performed by paired Student’s t test in accordance with the pre-defined statistical plan. Patients were dichotomized by the median Treg cell number, frequency and telomere length for analyses of LFS (log-rank test). LFS was defined as the time in days from start of immunotherapy with HDC/IL-2 to relapse or death from any cause using data available at the trial closing date (October 13, 2014), i.e., when patients had been followed-up for at least 24 months. Cox multivariable regression analysis that included age and number of induction cycles as potential confounders was utilized to further determine the impact of Treg distribution on LFS. Statistical analyses were performed using Graphpad Prism (Graph Pad Software, La Jolla, CA, USA) and IBM SPSS Statistics (IBM Corp., Armonk, NY, USA) software. All indicated p values are two-sided.
Results
Expansion of Tregs in blood during cycles of immunotherapy
Peripheral blood was drawn before and after the first and third 3-week cycle of HDC/IL-2 immunotherapy and analyzed for content of Tregs with Foxp3+CD25highCD4+ phenotype. A pronounced increase in the absolute numbers of blood Tregs (Fig. 1a, b) and in the percentage of Tregs among CD4+ cells (Fig. 4a) was observed during the first HDC/IL-2 treatment cycle. No significant changes in the absolute counts of Foxp3−CD4+ T cells were observed during treatment cycles (data not shown). Treg levels in blood contracted to baseline levels after completion of a treatment cycle and were again induced during subsequent treatment cycles albeit to a significantly lower extent (Figs. 1b, 4a).
Tregs expand during immunotherapy with HDC/IL-2. a Representative dot plots of Tregs (defined as Foxp3+CD25highCD4+) before (cycle 1, day 1, C1D1) and after (C1D21) the first HDC/IL-2 treatment cycle. b Box plots represent blood counts of Tregs before (D1) and after (D21) cycles 1 and 3 of immunotherapy (C1D1 n = 59, C1D21 n = 53, C3D1 n = 51, C3D21 n = 50, Student’s paired t test). c Patients were dichotomized by the median for low number of Tregs in black and high number of Tregs in red, at onset of immunotherapy (C1D1; left panel) or end of cycle 1 (C1D21; mid panel). The right panel shows the LFS of patients with low or high induction of Treg cell numbers during the first treatment cycle as analyzed by the log-rank test
In a first attempt to determine the impact of Treg levels on clinical outcome, patients were dichotomized by the median Treg count at onset of therapy (cycle 1, day 1; C1D1) or after the first treatment cycle (C1D21) followed by analysis of LFS. The Treg counts before or after the first treatment cycle did not predict LFS (Fig. 1c). Also, LFS did not differ between patients who were dichotomized based on high or low induction of Tregs during cycle 1 (Fig. 1c) or between patients in upper or lower quartiles of Tregs at onset or during therapy (p > 0.5, data not shown).
The majority of expanded Tregs show stable Foxp3 expression
To determine the origin and stability of the expanded Tregs, we analyzed the methylation status of the Treg-specific demethylated region (TSDR) in the FOXP3 gene locus in Tregs purified after a HDC/IL-2 treatment cycle. A demethylated promoter reflects a stable Foxp3 expression, which is characteristic of thymic-derived nTregs. The TSDR region in Tcons as well as in iTregs is, on the other hand, generally methylated [43, 46]. As shown in Fig. 2a, b, the TSDR in the FOXP3 gene locus of the expanded Tregs was predominantly demethylated. The accumulating Tregs thus showed stable Foxp3 expression and hence resembled nTregs, which was further supported by their expression of Helios (Fig. 2c), a marker proposed to identify nTregs [47].
Expanded Tregs resemble thymic-derived nTregs. a Methylation pattern of 15 CpG islands in the TSDR, located in the FOXP3 gene locus, for sorted Tcons from healthy donors (n = 2), sorted Tregs from healthy donors (n = 8) and sorted Tregs from Re:Mission patients (n = 9) with samples collected after treatment cycle 3 (C3D21). The color code indicates percentage methylation of each CpG island with yellow representing absence of methylation and blue 100% methylation. NA not analyzed. b Bars show the mean methylation of each CpG-site for healthy donors (n = 8) and Re:Mission trial patients (n = 9). Error bars display standard error of the mean (SEM). c Expression of Helios in Tregs before and after cycle 1 and cycle 3 of treatment with HDC/IL-2 (C1D1 n = 16, C1D21 n = 22, C3D1 n = 13, C3D21 n = 14). Statistical analyses were performed by Student’s paired t test
Immunosuppressive features of expanded Tregs
Numerous immunosuppressive features have been attributed to Tregs, including the constitutive expression of the inhibitory receptor CTLA-4 [48]. During cycles of HDC/IL-2, the expression of cell surface CTLA-4 on Tregs, but not on Tcons, was significantly increased followed by contraction to baseline levels between cycles (Fig. 3a, b). In line with the above-referenced findings for Treg induction (Fig. 1c), the expression level of CTLA-4 on Tregs did not significantly impact on patient outcome in terms of LFS (data not shown).
Expanded Tregs from Re:Mission trial patients are immunosuppressive. Median fluorescence intensity (MFI) of CTLA-4 on Tregs (a) and Tcons (b) in patient blood before and after treatment cycles 1 (C1D1 n = 19, C1D21 n = 25) and 3 (C3D1 n = 16, C3D21 n = 17). c Representative histograms of Tcon proliferation from a healthy donor and a Re:Mission patient. Black lines show the proliferation of Tcons in wells without Tregs and red shaded areas show proliferation of Tcons when Tregs were added in a ratio of 1:1. Division index (d) and proliferation index (e) are shown for Tcons from healthy donors (n = 5) and Re:Mission trial patients (n = 4) at the end of treatment cycle 3. Statistical analyses were performed by Student’s paired t test
We next determined whether the accumulating Tregs retained the immunosuppressive features of normal Tregs. To this end, Tregs (CD4+CD14−CD25hiCD127low) and Tcons (CD4+CD14−CD25lowCD127hi) were FACS-sorted from patient blood after a treatment cycle followed by assessment of the proliferation of anti-CD3/anti-CD28-stimulated Tcons in the presence or absence of Tregs. The patient-derived expanded Tregs reduced the proliferation of autologous Tcons as efficiently as did Tregs from healthy blood donors (Fig. 3c–e). Of note, the patient-derived Tcons proliferated more vigorously in response to anti-CD3/anti-CD28-stimulation compared with healthy donor Tcons (Fig. 3c, d), likely reflecting their primed status at the end of a HDC/IL-2 cycle.
Treg exhaustion and short Treg telomere length predict favorable clinical outcome
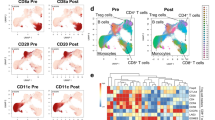
The analyses accounted for above indicated that the Tregs that accumulated during HDC/IL-2 immunotherapy did not negatively impact on clinical outcome despite showing features of immunosuppression. In addition to Tregs, NK cell counts were markedly increased in blood during treatment cycles of HDC/IL-2 (Fig. 4a, b). The favorable impact of aspects of NK cell biology on the outcome of patients in this trial is described in detail elsewhere [40, 41]. To further elucidate the reasons for the apparent inability of the accumulating Tregs to adversely affect patient outcome, we compared the kinetics of Treg and NK cell accumulation during immunotherapy. As shown in Fig. 4a, b, the magnitude of Treg induction, but not that of NK cell induction, was reduced in later treatment cycles. Furthermore, patients displaying high reduction in the fraction of Tregs at the end of cycle 3 compared with the end of cycle 1 showed significantly improved LFS (Fig. 4c). This difference remained significant in a multivariable analysis correcting for potential confounders for LFS (p = 0.025, Cox multivariable regression analysis).
Expansion of Tregs is reduced in later cycles of immunotherapy. Box plots display (a) the frequency of Tregs within the CD4+ compartment (C1D1 n = 59, C1D21 n = 63, C3D1 n = 52, C3D21 n = 53), and (b) frequency of NK cells as percentage of lymphocytes (C1D1 n = 62, C1D21 n = 63, C3D1 n = 53, C3D21 n = 53), before (D1) and after (D21) the first and third HDC/IL-2 treatment cycle. Statistical analyses were performed by Student’s paired t test. c Patients were dichotomized by the median for low (black) or high (red) reduction in Treg percentage from the end of cycle 1 to the end of cycle 3, and LFS was analyzed by the log-rank test. d Relative telomere length of Tregs FACS-sorted from patient blood obtained before and after the third treatment cycle or from healthy blood donors (Ctrl). e Kaplan–Meier plot comparing the LFS of patients with Treg telomere lengths on C3D21 below (black) and above (red) the median (log-rank test)
To clarify the mechanism underlying the decline in Treg induction during later treatment cycles, we set up an assay to determine telomere length by qPCR. Tregs were FACS-sorted from patient samples before and after treatment cycle 3 and analyzed for telomere length. The Treg telomere length did not differ significantly before and after a treatment cycle (Fig. 4d). However, short Treg telomeres at the end of treatment cycle 3 were significantly associated with reduced relapse risk (Fig. 4e).
Discussion
Upon diagnosis, AML patients receive induction chemotherapy aiming to achieve CR, which is defined as the microscopic disappearance of leukemic cells and the return of normal hematopoiesis. Despite additional courses of chemotherapy (consolidation), relapse in CR is common and significantly explains why the long-term survival of adult AML patients remains in the range of 30–40% [14]. A large body of evidence, including the graft-versus-leukemia reaction that mediates relapse prevention after allo-SCT, implicates functions of cytotoxic T cells and NK cells in controlling the malignant clone in AML [15, 39,40,41]. The purported role of cell-mediated immunity for the surveillance of leukemic cells in AML has inspired the development of immunotherapeutic strategies, in particular for patients in CR who harbor a minimal yet potentially life-threatening burden of leukemia (reviewed in [15]).
HDC/IL-2 is currently the only documented effective non-transplant immunotherapy for relapse prevention in AML beyond the chemotherapy phase [15, 32]. As the IL-2 component of this regimen may induce Tregs [35,36,37,38] the present study was designed to determine the magnitude of Treg induction during immunotherapy, the origin and function of accumulating Tregs and the potential impact of Tregs on relapse risk. We therefore analyzed serial blood samples from patients in first CR participating in the phase IV Re:Mission trial (n = 84) who received ten 3-week cycles of HDC/IL-2 after the completion of consolidation chemotherapy. The frequency of Tregs at the onset of immunotherapy was within or below the range in healthy subjects (3.1 ± 2.2% of CD4+ T cells; mean ± SD), which is in agreement with a recent study of AML patients in CR [49]. Treg counts increased considerably during cycles of HDC/IL-2, in particular during the first treatment cycle. At the end of the first cycle, Tregs typically comprised 15–25% of the CD4+ cell population in blood. These results concur with previous reports of Treg induction during treatment of cancer patients with IL-2 [35, 37, 38] and is likely explained by IL-2 acting via the high-affinity IL-2 receptor CD25 that is constitutively expressed by nTregs. However, randomized comparisons are required to exclude the possibility that the HDC component contributed to Treg induction. While we did not have access to bone marrow samples in this study, we reason that a similar increase in Treg counts is likely to occur also in the bone marrow, since the number of Tregs in blood and bone marrow were previously reported to be highly correlated [50].
We then asked whether the expanded population of Tregs showed stable or transient expression of Foxp3. In these cells, the TSDR in the FOXP3 gene locus was highly demethylated implying stable Foxp3 expression and suggesting that the reduction of Treg counts between cycles was explained by Treg apoptosis rather than the Tregs being reprogrammed into Tcons. Moreover, there was no increase in the number of Tcons during or between treatment cycles (data not shown). The thymus-derived nTregs are known to have a demethylated TSDR in the FOXP3 gene locus while this region generally is more methylated in iTregs. With the precaution that the TSDR region may become demethylated also in iTregs in response to antigen stimulation in the presence of IL-2 [51], we propose that the expanded Tregs were mainly derived from proliferating nTregs.
We observed that the Tregs accumulating at the end of a HDC/IL-2 treatment cycle expressed elevated levels of CTLA-4, which reportedly contributes to the immunosuppression exerted by these cells [48]. Also, the expanded Tregs suppressed the proliferation of Tcons in co-culture assays ex vivo. While it is conceivable that Treg induction may dampen the development of cell-mediated immunity of relevance to elimination of residual leukemia, our initial analysis did not reveal associations between the magnitude of Treg induction during initial cycles of immunotherapy and clinical outcome. It is conceivable, however, that the lack of association between Treg induction and clinical outcome may result from effects of HDC—a NOX2 inhibitor—on the immunosuppressive properties of Tregs. This possibility is supported by a previous study showing that immunosuppressive features of CD8+ Tregs rely on functional NOX2 [52]. However, monotherapy with IL-2 has been reported to increase Treg counts and limit the extent of graft-versus-host disease (GvHD) after allo-SCT in cancer patients, apparently without negatively affecting survival [35]. In accordance, results presented by Martelli et al. implied that allo-transplanted patients with acute leukemia who received donor-derived Tregs in conjunction with Tcons for protection against GvHD did not show increased relapse risk [53].
A more detailed analysis of Treg kinetics during treatment with HDC/IL-2 revealed that aspects of Treg function may indeed impact on clinical outcome. We observed that the magnitude of Treg induction was frequently blunted in later treatment cycles and that a reduced Treg accumulation in cycle 3 weakly but significantly prognosticated low relapse risk, thus supporting that sustained presence of Tregs may adversely impact on prognosis. In contrast, the induction of NK cells in blood remained largely stable throughout cycles of immunotherapy. The mechanisms underlying the different kinetics of NK cell and Treg induction should be further studied. However, in people over the age of 45 the supply of thymic nTregs is minimal and is sustained mainly by peripheral proliferation [54]. We thus speculate that the supply of nTregs may become exhausted during repeated cycles of immunotherapy, in contrast to the bone marrow supply of NK cells. In support of this assumption, we observed a significantly reduced accumulation of Tregs in later treatment cycle only in patients >45-years-old (Supplementary Fig. 1a).
The proliferation of normal somatic cells is limited by the length of telomeres, which typically progressively shorten with increasing age [55]. Accordingly, we observed a significant correlation between short Treg telomere length and age among the participating patients (Supplementary Fig. 1b). Despite high age being a dominant predictor of relapse risk in AML [56], short Treg telomeres at the end of a treatment cycle was observed mainly in older patients and significantly prognosticated favorable LFS. In agreement with the above-referenced hypothesis of Treg exhaustion during immunotherapy, we propose that short Treg telomere length may reflect a reduced capacity of nTregs to undergo proliferation and, hence, exert immunosuppression in subsequent treatment cycles.
While the preliminary nature of these findings should be emphasized, we speculate that immunosuppressive nTregs may be targeted for improved anti-leukemic efficacy of HDC/IL-2 immunotherapy. This view gains support from previous studies in which Tregs were targeted during immunostimulation with IL-2 in experimental leukemia using the combination of anti-CD25, aiming to deplete Tregs, and IL-2. This combination significantly improved the survival of leukemia-bearing mice over either treatment alone [57]. In further support for a role of Tregs in AML immunotherapy, Bachanova et al. reported that patients with relapsed or refractory AML showed encouraging CR rates and disease-free survival following depletion of host Tregs prior to the adoptive transfer of haploidentical NK cells and IL-2 [58]. Targeting Tregs, for example by use of antibodies blocking CTLA-4, may thus be considered in IL-2-based AML immunotherapy. An alternative approach to minimize a potential negative impact of Tregs may be to replace the IL-2 component with modified IL-2 variants or IL-15 that activate anti-leukemic effector cell populations with reduced or absent expansion of CD25high expressing Tregs [59,60,61].
Abbreviations
- Allo-SCT:
-
Allogeneic stem cell transplant
- AML:
-
Acute myeloid leukemia
- C1D1:
-
Cycle 1, day 1
- C1D21:
-
Cycle 1, day 21
- C3D1:
-
Cycle 3, day 1
- C3D21:
-
Cycle 3, day 21
- CR:
-
Complete remission
- GvHD:
-
Graft-versus-host disease
- HDC:
-
Histamine dihydrochloride
- iTregs :
-
Induced regulatory T cells
- LFS:
-
Leukemia-free survival
- NOX2:
-
Nicotinamide adenine dinucleotide phosphate oxidase isoform 2
- nTregs :
-
Natural regulatory T cells
- OS:
-
Overall survival
- qPCR:
-
Quantitative PCR
- ROS:
-
Reactive oxygen species
- Tcons :
-
Conventional T cells
- Tregs :
-
Regulatory T cells
- TSDR:
-
Regulatory T cell-specific demethylated region
References
Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK (2005) Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med 202:1375–1386
Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H (2005) Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 6:1219–1227
Yadav M, Stephan S, Bluestone JA (2013) Peripherally induced tregs—role in immune homeostasis and autoimmunity. Front Immunol 4:232
Haribhai D, Williams JB, Jia S et al (2011) A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 35:109–122
Wing K, Sakaguchi S (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 11:7–13
Lapierre P, Lamarre A (2015) Regulatory T cells in autoimmune and viral chronic hepatitis. J Immunol Res 2015:479703
Littwitz-Salomon E, Akhmetzyanova I, Vallet C, Francois S, Dittmer U, Gibbert K (2015) Activated regulatory T cells suppress effector NK cell responses by an IL-2-mediated mechanism during an acute retroviral infection. Retrovirology 12:66
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX (2005) Intratumor depletion of CD4 + cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med 201:779–791
Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC (2010) Regulatory T cells in cancer. Adv Cancer Res 107:57–117
Shang B, Liu Y, Jiang SJ, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3 + regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5:15179
Lai C, August S, Behar R, Polak M, Ardern-Jones M, Theaker J, Al-Shamkhani A, Healy E (2015) Characteristics of immunosuppressive regulatory T cells in cutaneous squamous cell carcinomas and role in metastasis. Lancet 385(Suppl 1):S59
Wolf D, Wolf AM, Rumpold H et al (2005) The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11:8326–8331
Dohner H, Weisdorf DJ, Bloomfield CD (2015) Acute Myeloid Leukemia. N Engl J Med 373:1136–1152
Martner A, Thoren FB, Aurelius J, Hellstrand K (2013) Immunotherapeutic strategies for relapse control in acute myeloid leukemia. Blood Rev 27:209–216
Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z, Xi X (2011) Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer 129:1373–1381
Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, Foon KA, Whiteside TL, Boyiadzis M (2009) Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res 15:3325–3332
Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS, Hirst WJ (2001) Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol 167:6021–6030
Costello RT, Sivori S, Marcenaro E et al (2002) Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 99:3661–3667
Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, Costello RT (2007) Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 109:323–330
Aurelius J, Thoren FB, Akhiani AA, Brune M, Palmqvist L, Hansson M, Hellstrand K, Martner A (2012) Monocytic AML cells inactivate antileukemic lymphocytes: role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood 119:5832–5837
Hellstrand K, Asea A, Dahlgren C, Hermodsson S (1994) Histaminergic regulation of NK cells. Role of monocyte-derived reactive oxygen metabolites. J Immunol 153:4940–4947
Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K (2000) Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood 96:1961–1968
Brune M, Hansson M, Mellqvist UH, Hermodsson S, Hellstrand K (1996) NK cell-mediated killing of AML blasts: role of histamine, monocytes and reactive oxygen metabolites. Eur J Haematol 57:312–319
Asea A, Hermodsson S, Hellstrand K (1996) Histaminergic regulation of natural killer cell-mediated clearance of tumour cells in mice. Scand J Immunol 43:9–15
Kolitz JE, George SL, Benson DM Jr et al (2014) Recombinant interleukin-2 in patients aged younger than 60 years with acute myeloid leukemia in first complete remission: results from Cancer and Leukemia Group B 19808. Cancer 120:1010–1017
Baer MR, George SL, Caligiuri MA et al (2008) Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: cancer and Leukemia Group B Study 9720. J Clin Oncol 26:4934–4939
Blaise D, Attal M, Reiffers J et al (2000) Randomized study of recombinant interleukin-2 after autologous bone marrow transplantation for acute leukemia in first complete remission. Eur Cytokine Netw 11:91–98
Pautas C, Merabet F, Thomas X et al (2010) Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 28:808–814
Lange BJ, Smith FO, Feusner J et al (2008) Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood 111:1044–1053
Mao C, Fu XH, Yuan JQ et al (2015) Interleukin-2 as maintenance therapy for children and adults with acute myeloid leukaemia in first complete remission. Cochrane Database Syst Rev 11:CD010248
Brune M, Castaigne S, Catalano J et al (2006) Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood 108:88–96
Campbell DJ (2015) Control of regulatory T cell migration, function, and homeostasis. J Immunol 195:2507–2513
Sakaguchi S, Miyara M, Costantino CM, Hafler DA (2010) FOXP3 + regulatory T cells in the human immune system. Nat Rev Immunol 10:490–500
Kennedy-Nasser AA, Ku S, Castillo-Caro P et al (2014) Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res 20:2215–2225
Kim N, Jeon YW, Nam YS, Lim JY, Im KI, Lee ES, Cho SG (2015) Therapeutic potential of low-dose IL-2 in a chronic GVHD patient by in vivo expansion of regulatory T cells. Cytokine 78:22–26
Koreth J, Matsuoka K, Kim HT et al (2011) Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365:2055–2066
Matsuoka K, Koreth J, Kim HT et al (2013) Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 5:179ra43
Sander FE, Rydström A, Bernson E et al (2016) Dynamics of cytotoxic T cell subsets during immunotherapy predicts outcome in acute myeloid leukemia. Oncotarget 7:7586–7596
Martner A, Rydström A, Riise RE, Aurelius J, Anderson H, Brune M, Foa R, Hellstrand K, Thoren FB (2016) Role of natural killer cell subsets and natural cytotoxicity receptors for the outcome of immunotherapy in acute myeloid leukemia. Oncoimmunology 5:e1041701
Martner A, Rydström A, Riise RE, Aurelius J, Brune M, Foa R, Hellstrand K, Thoren FB (2015) NK cell expression of natural cytotoxicity receptors may determine relapse risk in older AML patients undergoing immunotherapy for remission maintenance. Oncotarget 6:42569–42574
Dohner H, Estey EH, Amadori S et al (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453–474
Baron U, Floess S, Wieczorek G et al (2007) DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol 37:2378–2389
Bennett CL, Christie J, Ramsdell F et al (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27:20–21
Cawthon RM (2009) Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37:e21
Morikawa H, Sakaguchi S (2014) Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol Rev 259:192–205
Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM (2010) Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3 + T regulatory cells. J Immunol 184:3433–3441
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S (2008) CTLA-4 control over Foxp3 + regulatory T cell function. Science 322:271–275
Lichtenegger FS, Lorenz R, Gellhaus K, Hiddemann W, Beck B, Subklewe M (2014) Impaired NK cells and increased T regulatory cell numbers during cytotoxic maintenance therapy in AML. Leuk Res 38:964–969
Moon HW, Kim BH, Park CM, Hur M, Yun YM, Kim SY, Lee MH (2011) CD4 + CD25highFoxP3 + regulatory T-cells in hematologic diseases. Korean J Lab Med 31:231–237
Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM (2011) IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3 + T cells in vivo. J Immunol 186:6329–6337
Wen Z, Shimojima Y, Shirai T, Li Y, Ju J, Yang Z, Tian L, Goronzy JJ, Weyand CM (2016) NADPH oxidase deficiency underlies dysfunction of aged CD8 + Tregs. J Clin Invest 126:1953–1967
Martelli MF, Di Ianni M, Ruggeri L et al (2014) HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 124:638–644
Fessler J, Ficjan A, Duftner C, Dejaco C (2013) The impact of aging on regulatory T-cells. Front Immunol 4:231
Bär C, Blasco MA (2016) Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000Res. 5. eCollection 2016
Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J, Wheatley K, Hunter A, Russell N (2013) Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol 31:1293–1301
Hallett WH, Ames E, Alvarez M, Barao I, Taylor PA, Blazar BR, Murphy WJ (2008) Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol Blood Marrow Transpl 14:1088–1099
Bachanova V, Cooley S, Defor TE et al (2014) Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123:3855–3863
Arenas-Ramirez N, Woytschak J, Boyman O (2015) Interleukin-2: biology, design and application. Trends Immunol 36:763–777
Votavova P, Tomala J, Kovar M (2014) Increasing the biological activity of IL-2 and IL-15 through complexing with anti-IL-2 mAbs and IL-15Ralpha-Fc chimera. Immunol Lett 159:1–10
Perna SK, De Angelis B, Pagliara D et al (2013) Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin Cancer Res 19:106–117
Acknowledgements
The authors are indebted to the participating patients and to the participants in the Re:Mission Study Group. This work was supported by Meda Pharma, Bad Homburg, Germany (Study Sponsor), the Swedish Research Council, the Swedish Society for Medical Research (SSMF), the Swedish Cancer Society (Cancerfonden), the Swedish state via the ALF agreement, the Erna and Victor Hasselblad Foundation, the Torsten and Ragnar Söderberg Foundation, the Assar Gabrielsson Foundation, the Lars Hierta Memorial Foundation, Lion Cancer Foundation West, BioCARE—a National Strategic Research Program at University of Gothenburg, and the Sahlgrenska Academy at University of Gothenburg.
Author information
Authors and Affiliations
Contributions
Authors MB and RF were the principal investigators in the clinical trial. Authors FES, AS, KH, FBT and AM designed the research. Authors FES, MN, AR, JA, RER, CM, EB and RK performed and evaluated experiments. Authors FES, MN, AR, JA and AM analyzed the data. Authors FES, MN, KH and AM drafted the manuscript. All authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors Mats Brune and Kristoffer Hellstrand are past or present consultants to the study sponsor (Meda Pharma). Authors Frida Ewald Sander, Kristoffer Hellstrand, Fredrik B. Thorén and Anna Martner hold issued or pending patents protecting the use of histamine dihydrochloride in cancer immunotherapy. Authors Anna Martner, Robin Foà and Fredrik B. Thorén have received honoraria and/or travel grants from the study sponsor. Author Anders Ståhlberg declares stock ownership in TATAA Biocenter. The other authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sander, F.E., Nilsson, M., Rydström, A. et al. Role of regulatory T cells in acute myeloid leukemia patients undergoing relapse-preventive immunotherapy. Cancer Immunol Immunother 66, 1473–1484 (2017). https://doi.org/10.1007/s00262-017-2040-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2040-9