Abstract
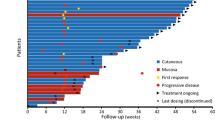
Melanomas in Chinese patients show relatively higher rates of acral and mucosal types than in other populations. However, the efficacy of checkpoint inhibitor therapies against these melanoma subtypes is not well defined. We analyzed 52 patients treated with ipilimumab, pembrolizumab, or a combination of both to evaluate the efficacy and safety of checkpoint inhibitors in Chinese patients with advanced melanoma, particularly those with acral and mucosal types. The objective response rates (ORRs) were 0, 25, and 20% for ipilimumab, pembrolizumab, and pembrolizumab plus ipilimumab, respectively. Pembrolizumab contained therapy was as effective in acral and mucosal melanoma patients (ORR 26.7 and 20%, respectively) as in non-acral cutaneous melanoma patients (ORR 26.7%). Baseline lactate dehydrogenase levels and relative lymphocyte counts were independent prognostic factors for PFS and OS. The incidences of grade 3–4 adverse events were 14% in the two monotherapy groups and 30% in the combined therapy group. The most frequent adverse events were elevation of aminotransferase, skin toxicity, thyroid dysfunction, pyrexia, and fatigue. Treatment-related rash or vitiligo was associated with a better prognosis. In summary, pembrolizumab-based therapy resulted in meaningful efficacy and good tolerability in Chinese patients with melanoma, including those with acral and mucosal types.


Similar content being viewed by others
Abbreviations
- ASTA:
-
Spartate aminotransferase
- ALT:
-
Alanine aminotransferase
- CSD:
-
Chronic sun-derived
- CR:
-
Completeremission
- CTCAE:
-
National Cancer Institute Common Terminology Criteria for Adverse Events
- DCR:
-
Disease control rate
- ECOG:
-
Eastern Cooperative Group
- Ipi:
-
Ipilimumab
- NR:
-
Not reached
- OS:
-
Overall survival
- ORR:
-
Objective response rate
- Pem:
-
Pembrolizumab
- PR:
-
Partial remission
- PFS:
-
Progression-free survival
- RECIST:
-
Response evaluation criteria in solid tumors
- RLC:
-
Relative lymphocyte count
- REC:
-
Relative eosinophil count
- SD:
-
Stable disease
- ULN:
-
Upper limit of normal
References
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM (2011) Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 16:5–24
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD (2015) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumabin unresectable or metastatic melanoma. J ClinOncol 33:1889–1894
Hamid O, Robert C, Daud A et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144
Topalian SL, Sznol M, McDermott DF et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J ClinOncol 32:1020–1030
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumaband ipilimumabor monotherapy in untreated melanoma. N Engl J Med 373:23–34
Long GV, Atkinson V, Cebon JS et al (2016) Pembrolizumab (pembro) plus ipilimumab (ipi) for advanced melanoma: Results of the KEYNOTE-029 expansion cohort. J ClinOncol 34(suppl 15):9506 (ASCO Annual Meeting abstract)
Chi Z, Li S, Sheng X, Si L, Cui C, Han M, Guo J (2011) Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer 11:85
Curtin JA, Fridlyand J, Kageshita T et al (2005) Distinct sets of genetic alterations in melanoma. N Engl J Med 353:2135–2147
Furney SJ, Turajlic S, Stamp G et al (2013) Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol 230:261–269
Furney SJ, Turajlic S, Stamp G et al (2014) The mutational burden of acral melanoma revealed by whole-genome sequencing and comparative analysis. Pigment Cell. Melanoma Res 27:835–838
Shoushtari AN, Munhoz RR, Kuk D et al (2016) The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 22:3354–3362
Wu P, Wu D, Li L, Chai Y, Huang J (2015) PD-L1 and survival in solid tumors: a meta-analysis. PLoS One 10:e0131403. doi:10.1371/journal.pone.0131403
Ribas A, Puzanov I, Dummer R et al (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16:908–918
Robert C, Ribas A, Wolchok JD et al (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384:1109–1117
Daud AI, Wolchok JD, Robert C et al (2016) Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 34:4102–4109
Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, Melero I, Ascierto PA (2016) Cancer treatment with anti-PD-1/PD-L1 agents: Is PD-L1 expression a biomarker for patient selection? Drugs 76:925–945
Madore J, Vilain RE, Menzies AM et al (2015) PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell. Melanoma Res 28:245–253
Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J (2016) Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer 114:256–261
Weide B, Martens A, Hassel JC et al (2016) Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. doi:10.1158/1078-0432.CCR-16-0127
Hua C, Boussemart L, Mateus C et al (2016) Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 152:45–51
Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S (2015) Pembrolizumabcutaneous adverse events and their association with disease progression. JAMA Dermatol 151:1206–1212
Ferrucci PF, Ascierto PA, Pigozzo J et al (2016) Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 27:732–738
Boutros C, Tarhini A, Routier E et al (2016) Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13:473–486
Wen X, Wang Y, Ding Y, Li D, Li J, Guo Y, Peng R, Zhao J, Zhang X, Zhang XS (2016) Safety of immune checkpoint inhibitors in Chinese patients with melanoma. Melanoma Res 26:284–289
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received support from the National Natural Science Foundation of China (Grant No. 81272341).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The institutional review board at our hospital approved this study.
Informed consent
All patients provided written informed consent for this study.
Additional information
X. Wen and Y. Ding contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, X., Ding, Y., Li, J. et al. The experience of immune checkpoint inhibitors in Chinese patients with metastatic melanoma: a retrospective case series. Cancer Immunol Immunother 66, 1153–1162 (2017). https://doi.org/10.1007/s00262-017-1989-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1989-8