Abstract
The natural adjuvant properties of bacterial ghosts (BGs) lie within the presence of intact pathogen-associated molecular patterns on their surface. BGs can improve the direct delivery, natural processing and presentation of target antigens within dendritic cells (DCs). Moreover, sensitization of human DCs by cancer cell lysate (oncolysate)-loaded BGs in the presence of IFN-α and GM-CSF enhanced DC maturation as indicated by an increased expression of maturation markers and co-stimulatory molecules, higher production of IL-12p70 and stimulation of significantly increased proliferation of both autologous CD4+ and CD8+ T cells compared to DCs matured in the presence of purified lipopolysaccharide. The induced T cells efficiently recognized oncolysate-derived tumor-associated antigens expressed by cancer cells used for the production of oncolysate. Our optimized one-step simultaneous antigen delivery and DC maturation-inducing method emerges as a promising tool for the development and implementation of next-generation cellular cancer immunotherapies.
Similar content being viewed by others
Introduction
Despite the renaissance of cancer immunotherapy over the past two decades and advances in cancer prophylaxis and therapeutic approaches, malignant diseases still represent a major health problem in all parts of the world and their incidence, which according to the WHO/IARC World Cancer Report 2014 will increase by 57% worldwide in the next 20 years [1]. Standard cancer treatment strategies, including surgery, chemotherapy, radiotherapy and targeted therapies, generally do not guarantee long-lasting disease control in the majority of patients, especially at an advanced stage of disease. There is ample evidence that the immune system, especially Th1-type cytotoxic immune responses, plays a critical role in the control of cancer growth and is crucial for the therapeutic activity of current standard cancer treatment approaches [2, 3]. However, cancer cells exhibit a high capacity of escape from immunosurveillance, significantly contributing to the failure of cancer treatment and relapse or progression of the disease [4, 5]. Therefore, cancer-dysbalanced anti-tumor immunity should be restored by therapeutic means known as tumor immunotherapy. Tumor immunotherapy aims to (re)stimulate the patient’s immune system by building up functionally competent immune responses against patient-specific TAAs and reverting the immunosuppressive tumor microenvironment, thereby leading to immune-mediated cancer control, which may manifest as either a complete or partial elimination of residual cancer cells or a durable stable disease [6].
Therapeutic cancer vaccination is one of the most promising immunotherapeutical approaches and exploits dendritic cells (DCs) to mediate its desirable impact on anti-tumor immune responses and the resulting clinical activity. DCs are the most potent professional antigen-presenting cells (APCs) that have outstanding immunostimulatory and immunomodulatory properties and play a key role in maintaining a delicate balance between active immunity and immune tolerance [7]. A unique capacity of DCs to prime anti-tumor immune responses place them in a prominent position in therapeutic tumor vaccine development and tumor immunotherapy in general [8]. The possibility of generating functionally competent DCs ex vivo from different precursors led to the development of various DC production protocols. DC-based therapeutic cancer vaccines have been tested in numerous clinical trials [9]; however, only one professional APC-based cancer immunotherapy, Sipuleucel-T (Provenge), was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of metastatic castration-resistant prostate cancer [10]. Several phase III clinical trials evaluating the therapeutic potential of DCs are currently underway (www.clinicaltrials.gov; accessed 20 August 2016). Different approaches using various Toll-like receptor (TLR) agonists, cytokines and their combinations to induce proper DC maturation ex vivo were generated in the past decades and are currently under intensive clinical investigations [8, 11–16]. However, the recently approved Provenge and some previously clinically tested DC vaccines, which were generated using various maturation protocols, did not meet the high expectations of DC-based therapeutic vaccines. These facts clearly point toward the need for optimization of protocols to generate clinical-grade DCs capable of inducing effective anti-tumor immune responses that lead to tumor elimination, regression or durable stabilization of the disease.
In a recent report, a one-step protocol for monocyte-derived DC maturation and loading with TAAs employed lentiviral transduction of DCs. Monocytes were directly induced to self-differentiate into tumor-antigen-loaded DCs upon transduction with lentivirus encoding for the cytokines granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 and a melanoma antigen tyrosinase-related protein 2 [17]. Another group has recently reported a study describing the transduction of melanoma cells (source of TAAs) with costimulatory molecules that are essential for adequate T cell activation, thereby providing tumor cells with properties of professional APCs and enabling them to directly activate CTLs without the participation of DCs and involvement of CD4+ T helper cells in the activation process [18]. We have shown previously that empty non-living bacterial cell envelopes, known as bacterial ghosts (BGs), possess intact surface structures, such as lipopolysaccharide (LPS), flagellin, peptidoglycan and many others, that are ligands for various pattern recognition receptors (PRRs) and are collectively known as pathogen-associated molecular patterns (PAMPs) [19]. BGs can stimulate strong cellular and humoral immune responses against BGs themselves and heterologous antigens carried by BGs [19]. Moreover, we demonstrated that BGs activate and mediate the maturation of DCs and can deliver plasmid DNA encoding target antigens into DCs [20, 21]. Proper expression of delivered genes within the cytosol of DCs led to their natural processing and presentation and stimulation of an efficient antigen-specific immune response [20].
Activation of an anti-tumor immune response leading to elimination of all tumor deposits and distant metastases requires TAAs either released directly from cancer cells or delivered exogenously, presentation of TAAs by fully mature DCs, stimulation of TAA-specific T cells capable of crossing protection barriers formed around the tumor microenvironment and recognition and killing of cancer cells. Stimulation of an anti-tumor immune response with a single antigen does not meet all the requirements for tumor eradication, but these requirements can be achieved using multiple antigen stimulation. Multi-TLR targeting and activation by BGs capacitate natural processing and presentation of delivered TAAs and induce competent maturation of DCs capable of eliciting effective TAA-specific immune responses. Cancer cell lysate (oncolysate)-loaded BGs serve as a convenient system for loading DCs with multiple undefined TAAs and inducing their proper maturation in a one-step process, which makes the clinical-grade DC generation process more standardized, straightforward and cost-effective. Combining BGs with numerous TAAs present within the oncolysate generated from a patient’s tumor would significantly enhance the chances of stimulating effective qualified cytotoxic T cells.
Here, we report the capacity of BGs to enhance the delivery of TAAs present within the oncolysate to DCs and induce their maturation ex vivo, overcoming the potential influence of the immunosuppressive tumor milieu [22, 23]. Our data show that polarization of human monocyte-derived DCs in the presence of BGs combined with IFN-α led to an enhanced secretion of IL-12p70 and stimulation of autologous T cells capable of recognizing native cancer cells used for the preparation of the oncolysate.
Materials and methods
Production of bacterial ghosts
BGs from E. coli Nissle 1917 were produced by the controlled expression of the phage-derived lysis protein E as described previously [24]. For safety reasons to fully inactivate all residual non-lysed viable bacterial cells and DNA present in the BG suspension, the BG preparation was treated with two equal doses of β-propiolactone (Ferak, Berlin, Germany) at 30-min intervals followed by extensive washing with demineralized sterile water by diafiltration [24]. The washed product was dispensed into aliquots, frozen at −80 °C and lyophilized. Dry-powdered product was stored at room temperature (RT) until further use.
Preparation of oncolysate
The T98G-glioblastoma cell line (European Collection of Cell Cultures, Cat. No. 92090213, a Health Protection Agency Culture Collection, Salisbury, UK) was maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, 1 mM sodium pyruvate and 0.1 mM nonessential amino acids solution (all obtained from Gibco, Invitrogen, Carlsbad, CA) in a humidified 5% CO2 incubator at +37 °C. Cancer cells were harvested by trypsinization, washed twice with DPBS and resuspended in complete DC culture medium (1 × 107/mL). Oncolysates were prepared from cancer cell suspension by six cycles of freeze-thawing in a mixture composed of dry ice and methanol (Sigma-Aldrich, Irvine, UK). Subsequently, the cell lysate was sonicated in an ultrasonic bath for 5 min. Cell debris together with the cell lysate was collected in one tube, centrifuged at 15,000 rpm for 10 min followed by quick and careful collection of the supernatant (oncolysate) without a pellet using a fine needle. Non-filtrated cell lysates were aliquoted and stored at −80 °C until further use.
Generation of monocyte-derived DCs
Immature monocyte-derived DCs were generated from peripheral blood mononuclear cells (PBMCs) obtained from buffy-coats of healthy donors at the Department of Transfusion Medicine and Blood Bank, University Hospital Brno, Brno, Czech Republic. The study was approved by the Ethical Committee of the University Hospital Brno. PBMCs were separated by density gradient centrifugation on Histopaque (Sigma), resuspended in CellGro DC medium (CellGenix, Freiburg, Germany) supplemented with 50 µg/mL DNase I (Roche, Mannheim, Germany), and allowed to adhere to the surface of 75 cm2 culture flasks (Corning, Sigma) for 2 h at +37 °C in a humidified 5% CO2 atmosphere. After washing, adherent monocytes were cultured for 3 days in culture medium supplemented with IFN-α2a (IFN-α; 3000 IU/mL; Roferon A, Roche) and recombinant human GM-CSF (rhGM-CSF; 1000 IU/mL; mGMP-rHuGM-CSF, clinical grade, Gentaur, Kampenhout, Belgium). No serum or antibiotics were added. On day 4, immature DCs (iDCs) were collected, transferred into 6-well plates (Corning) containing complete culture medium (CellGro; IFN-α2a, 3000 IU/mL; rhGM-CSF, 1000 IU/mL) and incubated for 4 h at +37 °C in a humidified 5% CO2 atmosphere with the following maturation stimuli: a T98G-glioblastoma cell line lysate (oncolysate) alone (oncolysate applied to DCs at a ratio of cancer cells [Oncolysate]/DCs—3:1) (1), an oncolysate plus LPS (200 ng/mL, Calbiochem, Merck Millipore, Darmstadt, Germany) (2) or a freshly prepared mixture of oncolysate and BGs (3). The solution was made by mixing oncolysate (cancer cells/DCs—3:1) with a lower amount of BGs (BGs/DCs—10:1) or a higher amount of BGs (BGs/DCs—100:1) and incubated at RT for 60 min with gentle shaking. Then, the mixture was immediately added to immature DCs. After co-culture, the DCs were washed to remove the excess stimuli and incubated in complete culture medium for 48 h.
DC phenotyping
Mature DCs were harvested 48 h after the incubation period with maturation stimuli, washed with FACS buffer [3% heat inactivated human AB serum (Sigma), 0.1% BSA (Sigma), 0.01% NaN3 (Sigma) in PBS (Invitrogen)] and surface stained with fluorescence-labeled mAb. The antibodies used included CD80, CD86, HLA-DR, CD56 (Beckman Coulter, Fullerton, CA, USA), CD83 and CD14 (BD Biosciences, San Jose, CA, USA), CD1a and CD11c (Exbio, Vestec, Czech Republic) and CCR7 (R&D Systems, Minneapolis, MN, USA). Propidium iodide (PI; Sigma) was added immediately before analysis to assess cell viability and exclude dead cells. The cells were analyzed by flow cytometry on a FACSCanto II Flow Cytometer using BD FACSDiva Software (both BD Biosciences).
T cell proliferation assay
The stimulatory capacity of generated DCs was determined in a mixed leukocyte reaction. Autologous or allogeneic T cells (1 × 105) were labeled with carboxyfluorescein succinimidyl ester (CFSE; 2.5 µM; Invitrogen) for 10 min at +37 °C in a humidified 5% CO2 atmosphere and incubated with DCs matured with the different stimuli (DCs/T cells ratio of 1:10) in 96-well flat bottom plates in X-VIVO 10 (Lonza, Verviers, Belgium) supplemented with 5% heat inactivated human AB serum. T cells incubated alone and with phytohemagglutinin (PHA; 5 µg/mL; Sigma) served as negative and positive controls, respectively. After 6 days of incubation, the cells were collected and surface stained with fluorochrome-labeled CD3 (Beckman Coulter), CD4 (Exbio) and CD8 (Exbio) mAbs. Proliferation of responder cells was analyzed by flow cytometry measuring the CFSE levels (fluorescence “dilution” related to mitotic cell division) in T cells.
Cytokine secretion assay
Culture supernatants were collected 24 h and 48 h after adding of maturation stimuli to immature monocyte-derived DCs. Cytokine levels of IL-12p70, IFN-γ, TNF-α, IL-6, IL-10 and IL-1β in the supernatants were detected using a Human FlowCytomix Kit (Bender Medsystems GmbH, Vienna, Austria) on a FACSArray Bioanalyzer (BD Biosciences) according to the manufacturer’s instructions.
T cell cytotoxicity assay
Autologous T cells were sensitized by DCs matured with the different stimuli. After 6 days of culture in X-VIVO 10 medium supplemented with 5% heat inactivated human AB serum, T cells (effectors) were collected, washed and added to 1 × 105 CFSE-labeled target cancer cells (T98G) at the ratio of effectors/targets—10:1, and incubated for 24 h. Cancer cells incubated without pre-stimulated effector cells served as controls for spontaneous cell death (negative control). Lysis of cancer cells after incubation with 10% ethanol served as a positive control. The efficacy of recognition and killing of tumor cells by T cells was calculated using the following formula: [(a − b)/(c − b)] × 100 (where a = sample; b = negative control; c = positive control). PI was added immediately before measurement to detect cell viability and dead cells. Cells were analyzed by flow cytometry.
Statistical analysis
Experimental data were analyzed by GraphPad Prism 5 (GraphPad Software, La Jolla, CA) and Statgraphics Centurion XVII (Statpoint Technologies, Inc., Warrenton, VA). The one-way analysis of variance (ANOVA) test was used to determine individual significant differences between the means of the analyzed groups. Differences were considered significant with a P value <0.05.
Results
DC generation and characterization
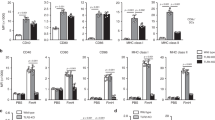
We have previously demonstrated that BGs generated from E. coli NM522 and M. haemolytica promote the maturation of DCs in the presence of a maturation cocktail composed of GM-CSF, IL-4, TNF-α, IL-1β, IL-6 and PGE2 known as the Jonuleit cocktail [25] and are able to deliver heterologous genes to the cytosol of DCs resulting in their expression with a transfection efficacy up to 85% [21]. To shorten the DC generation protocol required for therapeutic applications, exclude the controversial impact of PGE2 [9, 26] and improve the immunostimulatory capacity of DCs, we hypothesized that generation of immature DCs from peripheral blood monocytes in the presence of GM-CSF and IFN-α followed by multi-TLR activation mediated by BGs loaded with oncolysate should yield fully potent mature DCs presenting TAAs processed from internalized oncolysate. To test this hypothesis, immature DCs were generated from peripheral blood monocytes after 3 days of incubation in the presence of GM-CSF and IFN-α. Maturation of immature monocyte-derived DCs was induced using various stimuli, including oncolysate alone (1), oncolysate combined with LPS (2) or freshly prepared mixture of oncolysate preincubated with E. coli Nissle 1917 BGs [10 per cell (3) or 100 per cell (4)]. After a short (4 h) DC activation with these maturation stimuli, DCs were washed and incubated for an additional 2 days in complete medium containing GM-CSF and IFN-α. The viability of the cells was assessed immediately prior to analysis by adding PI (Fig. 1b). DCs generated in the presence of oncolysates supplemented with BGs had an overall comparable immunophenotype profile with a significantly increased expression of DC maturation marker CD83 and co-stimulatory molecule CD86 (Fig. 1a). In addition, pure LPS and a higher amount of E. coli Nissle 1917 BGs (100 BGs per cell) mediated lower expression of CD14 on DCs compared to oncolysate alone and a reduced number of E. coli Nissle 1917 BGs per cell (10/cell); however, this expression was not statistically significant (Fig. 1a). Although a decreased expression of CD1a, CD11c, HLA-DR and CD56 maturation markers was detected on DCs stimulated with oncolysate plus LPS (2) and oncolysate-loaded BGs [10 per cell (3) or 100 per cell (4)] compared with DCs matured by oncolysates alone (1), the difference was not statistically significant (Fig. 1b). Collectively, these results indicate similar maturation-inducing effects mediated either by a single TLR agonist (LPS) or by the empty intact shells of bacteria containing multiple PAMPs. Moreover, our data confirmed the fact that TAAs and other cancer cell’s constituents present within oncolysate are not capable of inducing and completing the process of proper DCs maturation (characterized by the surface expression of CD83 and CD80) without the “help” of competent external stimuli.
DC surface marker expression. a, b Immature DCs were prepared by incubation of monocytes obtained from peripheral blood of normal healthy donors in CellGro DC medium supplemented with IFN-α (3000 IU/mL) and rhGM-CSF (1000 IU/mL) for 3 days. Maturation markers of DCs were analyzed by multicolor flow cytometry 48 h after a short (4 h) stimulation of immature DCs with oncolysate obtained from the glioblastoma cell line T98G and BGs from E. coli Nissle 1917 (10 and 100 BGs/1DC) in the presence of IFN-α and rhGM-CSF. Immature DCs incubated with IFN-α, rhGM-CSF and oncolysate supplemented with LPS (200 ng/mL) or without extra maturation stimuli served as controls. The y axis represents the percentage of cells expressing specific differentiation antigen. The viability of the cells was assessed by adding PI immediately prior to the analysis. Box-and-whisker plots of the data obtained in four independent experiments from different normal healthy donors are shown. Boxes represent interquartile ranges; the horizontal bar within each box indicates the median; whiskers show the minimum and the maximum. P values <0.05 were considered significant and are indicated with asterisks (*P < 0.05; **P < 0.01)
Oncolysate-loaded BGs significantly modulate cytokine profile of DCs
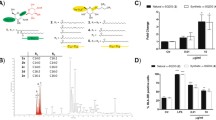
Cytokine profiling of DCs activated by either oncolysate alone or combined with bacteria-derived stimuli was performed by measuring the levels of Th1 polarizing (IL-12p70, IFN-γ), pro-inflammatory (TNF-α, IL-1β, IL-6) and immunosuppressive (IL-10) cytokines (Fig. 2). Cytokine levels were analyzed in culture supernatants obtained 24 and 48 h after short (4 h) DC incubation with the investigated maturation/activation stimuli. Increased production of all analyzed cytokines was detected in the supernatants of DCs activated by oncolysate supplemented with the tested bacterial stimuli but not in those stimulated by oncolysate alone. After 24 h of incubation, only DCs activated by oncolysate supplemented with the higher amount of E. coli Nissle 1917 BGs (100 BGs per cell) showed a significantly increased production of IL-12p70, IFN-γ, TNF-α, IL-6, IL-10 and IL-1β (Fig. 2). Except for IL-1β production (Fig. 2f), elevated cytokine production levels were also detected 48 h after activation with the same stimuli (100 BGs per cell). In addition, an elevated production of IL-10 by DCs activated with oncolysates mixed with the lower amount of E. coli Nissle 1917 BGs (10 BGs per cell) was observed; however, the expression was statistically significant only in culture medium collected 24 h after DC activation (Fig. 2e). Moreover, the addition of LPS and both investigated amounts of E. coli Nissle 1917 BGs to oncolysate significantly increased production of IL-6 by DCs as detected 24 and 48 h after stimulation compared to activation with oncolysate alone (Fig. 2d). A significant increase in IL-12p70, IFN-γ and TNF-α levels was detected only in supernatants from cultures of DCs activated by oncolysates mixed with a higher amount of E. coli Nissle 1917 BGs (100 BGs per cell) both at 24 h and 48 h of incubation (Fig. 2a, b, c). DCs stimulated with oncolysate alone and that were missing extra maturation stimuli produced very low amounts of both IFN-γ and IL-1β, while the levels of IL-12p70, TNF-α, IL-6 and IL-10 were almost undetectable (Fig. 2).
Cytokine profile of DCs matured in the presence of oncolysate and different bacterial stimuli. Immature DCs were activated with IFN-α, rhGM-CSF and oncolysate obtained from the glioblastoma cell line T98G for short time (4 h) in the presence of pure LPS (200 ng/mL) or BGs from E. coli Nissle 1917 (10 and 100 BGs/1DC) prior to measuring cytokine production in supernatants collected after 24 h (white bars) and 48 h (gray bars) incubations. Cells activated with oncolysate alone and without additional bacterial stimuli served as negative controls. The levels of cytokines released from DCs—a IL-12p70, b IFN-γ, c TNF-α, d IL-6, e IL-10 and f IL-1β were measured using a FACSArray Bioanalyzer. Box-and-whisker plots of the data obtained in four independent experiments from different normal healthy donors are shown. Boxes represent interquartile ranges; the horizontal bar within each box indicates the median; whiskers show the minimum and the maximum. P values <0.05 were considered significant and are indicated with asterisks (*P < 0.05; **P < 0.01; ***P < 0.001)
Activation and maturation of DCs by oncolysate-loaded BGs improved their capacity to stimulate the proliferation of autologous T cells
The ability of the analyzed DC populations to stimulate T cell proliferation was assessed by one-way allogeneic and autologous mixed lymphocyte reactions (MLR) using fluorescence-labeled T cells as responder cells. Proliferation of total T cells (CD3+), helper (CD3+CD4+) and cytotoxic (CD3+CD8+) T cells was measured by flow cytometry after 6 days of incubation with the tested DC populations at the cell ratio DCs/T cells—1:10. The gating strategy to determine T cell proliferation and a representative histogram data set of the CFSE-stained T cells are depicted in Supplementary Figures 1 and 2, respectively. T cells incubated in the presence of PHA and DCs activated with oncolysate alone served as positive and negative controls, respectively. All the examined DC populations elicited a robust response of allogeneic T cells (both CD3+CD4+ and CD3+CD8+ T cells) confirmed by increased proliferation, but no significant difference in stimulatory capacity of the tested DC populations was detected (Fig. 3a). Contrary to the results obtained from allogeneic MLR, autologous MLR revealed exceptional stimulatory capacities of DCs matured with oncolysate-loaded E. coli Nissle 1917 BGs (both 10 and 100 BGs per cell). DCs activated with oncolysate-loaded E. coli Nissle 1917 BGs induced significantly higher autologous T cell proliferation compared with DCs matured with oncolysate alone or oncolysate supplemented with LPS. Moreover, there was no significant difference in autologous T cell proliferation induced by DCs activated with oncolysate plus LPS versus DCs activated with oncolysate alone. Interestingly, DCs activated with oncolysate-loaded BGs elicited proliferation of both CD3+CD4+ and CD3+CD8+ autologous T cells at a similar level, while the number of proliferating autologous CD3+CD4+ T cells was more than threefold higher than that of CD3+CD8+ T cells after stimulation with DCs matured in the presence of oncolysate either with or without LPS (Fig. 3b). These results demonstrated that the activation and maturation of DCs in the presence of oncolysate-loaded E. coli Nissle 1917 BGs followed by DCs incubation with IFN-α and rhGM-CSF led to the generation of potent DCs capable of eliciting strong proliferation of both allogeneic and autologous CD4+ and CD8+ T cells. Moreover, data shown in Fig. 3 clearly indicate high functional competence of DCs matured in the presence of oncolysate-loaded BGs, thereby highlighting the natural adjuvant potential of BGs and their capacity to target multiple PRRs expressed by DCs.
Allogeneic and autologous immunostimulatory capacities of analyzed DCs. A short incubation of immature DCs with IFN-α, rhGM-CSF and oncolysate obtained from the glioblastoma cell line T98G and E. coli Nissle 1917 BGs (4 h) significantly enhanced the capacity of DCs to stimulate the proliferation of autologous T cells compared to DCs matured with IFN-α, rhGM-CSF and oncolysate supplemented either with pure LPS or without extra maturation stimuli. Stimulatory capacities of analyzed DC populations were determined after 6 days of incubation in the presence of CFSE-labeled allogeneic or autologous T cells at the ratio of DCs/T cells—1:10. Stimulated cells were stained after incubation with a panel of monoclonal antibodies (anti-CD3, anti-CD4 and anti-CD8), and proliferation of both allogeneic and autologous T cells was determined by multicolor flow cytometry. The values were calculated as percentage of cells proliferated spontaneously subtracted from the percentage of cells proliferated after stimulation with distinct populations of DCs. T cells incubated with PHA (5 µg/mL) served as a positive control. a Allogeneic immunostimulatory capacities of analyzed DC populations. b Autologous immunostimulatory capacities of analyzed DC populations. Box-and-whisker plots of data obtained in four independent experiments from different normal healthy donors are shown. Boxes represent interquartile ranges; the horizontal bar within each box indicates the median; whiskers show the minimum and the maximum. P values <0.05 were considered significant and are indicated with asterisks (*P < 0.05; **P < 0.01; ***P < 0.001)
DCs activated by oncolysate-loaded BGs elicit the generation of autologous cytotoxic T cells capable of recognizing natural TAAs
It was important to determine whether autologous T cells stimulated by DCs activated and matured by oncolysate-loaded BGs can recognize natural TAAs expressed by cancer cells used to prepare the oncolysate. To test this hypothesis, we collected T cells (effector cells; E) sensitized with differentially matured DCs for 6 days and mixed them with CFSE-labeled T98G cancer cells (target cells; T) at a ratio of E/T, 10:1. The gating strategy to determine of T cell cytotoxicity and killing of cancer cells and a representative histogram data set of the CFSE-stained cancer cells are depicted in Supplementary Figures 3 and 4, respectively. T98G cancer cells, serving as target cells, were used to prepare oncolysate as a source of TAAs. After 24 h of incubation, the target T98G cancer cell recognition and killing efficacy of induced tumor-specific T cells were determined by flow cytometry. A tumor recognition assay confirmed the previous results, showing that BGs loaded with oncolysate possess a superior ability to activate DCs, modulate their cytokine profile and the capacity to elicit robust T cell response. The strongest capacity to recognize native antigens naturally presented by cancer cells was detected using T cells stimulated by DCs activated and matured in the presence of oncolysate supplemented with both tested amounts of E. coli Nissle 1917 BGs. Increased tumor recognition efficacy by stimulated T cells was also detected after using pure LPS as an adjuvant mixed with oncolysate for DC activation; however, a significantly increased killing efficacy was only detected when oncolysate was supplemented with BGs (Fig. 4). Interestingly, a lower number of BGs were used together with oncolysate for activation and maturation of DCs (10 BGs per 1 DC) compared to both a higher amount of BGs (100 BGs per 1 DC) and LPS has a greater impact on stimulation of the cytotoxic T cells possessing enhanced killing potency. Moreover, these results clearly showed that the use of multi-TLR agonists (monophosphoryl lipid A, LPS, flagellin and peptidoglycan) containing BGs [19] compared to a single TLR agonist (LPS) leads to the stimulation of T cells with a significantly higher killing efficacy. The results indicate that T cells elicited by BGs-oncolysate-DCs represent a spontaneous polyclonal population of effector cells due to the source of TAAs originating from the glioblastoma cancer cell line used in the study and PBMCs obtained from healthy donors. Collectively, these data demonstrate the efficacy of DCs activated by oncolysate and BGs to elicit a robust in vitro tumor-antigen-specific T cell response comprising fully functional cytotoxic T cells.
Recognition of cancer cells and cytotoxic effects of autologous T cells induced by DCs activated with oncolysates and bacteria-derived stimuli. Autologous T cells were incubated for 6 days in the presence of DCs briefly (4 h) activated with oncolysates prepared from T98G cancer cells combined with pure LPS (200 ng/mL), BGs from E. coli Nissle 1917 (10 and 100 BGs/1DC) or without extra maturation stimuli. Subsequently, stimulated T cells (effector cells; E) were added to fresh CFSE-labeled T98G cancer cells (target cells; T) at the ratio of E/T—10:1. Specific lysis of cancer cells was determined 24 h after mutual co-incubation by flow cytometry. The percentage of tumor recognition and killing efficacy was calculated as described in the Materials and methods. Box-and-whisker plots of data obtained in four independent experiments from different normal healthy donors are shown. Boxes represent interquartile ranges; the horizontal bar within each box indicates the median; whiskers show the minimum and the maximum. P values <0.05 were considered significant and are indicated with asterisks (*P < 0.05; **P < 0.01)
Discussion
Over the past two decades, a high number of protocols generating clinical-grade DCs ex vivo were developed [9]. While the results from the very first clinical studies did not completely fulfill the high expectations that were placed on DC therapy in the past, recently published data show that great progress has been made in therapeutic DC vaccine development and their use in cancer immunotherapy with minimal side effects [12]. To date, more than 500 therapeutic DC vaccination clinical studies have been registered at ClinicalTrials.gov, a service of the US National Institutes of Health (www.clinicaltrials.gov; accessed 20 August 2016), providing new insights into DC-based cellular therapies [8]. However, a universally acceptable protocol has not yet been defined and approved also due to the missing data from properly designed and standardized clinical studies investigating the efficacy of a defined DC vaccine for the treatment of patients with reduced variables [13]. Moreover, the production of DC vaccines belongs to the agenda of advanced therapy medicinal products supervised by EMA. Due to the requirements for DC vaccine production, which are excessively time-consuming and high-priced and are slowing the process of their use in the clinic, great efforts have been made to optimize and standardize the DC vaccine manufacturing process and improve their immunostimulatory properties compared to the first-generation DC vaccines.
Our novel approach for the generation of mature DCs from blood monocytes is based on an innovative one-step (simultaneous) antigen delivery and DC maturation-inducing platform that exploits oncolysate-loaded BGs in the presence of GM-CSF and IFN-α. BGs have bacterial bio-adhesive surface properties and immunostimulating components in their original state [27]. We have shown previously that BGs are well recognized by cells expressing TLRs, including professional APCs and cancer cells, and that they are capable of delivering both DNA and protein antigens to target cells [19–21, 28–30]. The use of BGs provides an opportunity to target multiple PRRs, rather than one or several selected PRRs, expressed by DCs. Ligation of various PRRs triggers natural processing and presentation of TAAs, DC maturation, secretion of Th1-type cytokines and stimulation of effector tumor-antigen-specific T cells [7]. For example, after intravenous immunization of mice with DCs transfected ex vivo with BGs loaded with target antigen-encoding plasmid DNA, antigen-specific CD4+ and CD8+ T cell immune responses were generated [20].
We and others utilize lysates prepared from cancer cells (oncolysate) as a source of various “own” non-defined polyvalent TAAs [31–33] combined with TLR targeting-mediated activation of DCs [15, 16, 34–36]. The major advantage of using oncolysate prepared from either a cancer cell line or a sample of whole autologous tumor lies in the presence of multiple unique “real-time” TAAs expressed by malignant cells. Autologous tumor cells possess individual patient-specific mutational antigens (neo-antigens) that appear as a result of random point mutations in various genes. Some of these non-defined neo-TAAs may serve as the most accurate targets for the effectors of an anti-tumor immune response, mainly cytotoxic T cells. Moreover, the use of individual unique TAAs should also minimize the effect of regulatory cells, thereby improving the efficacy of the induced immune response [37]. Implementation of the BG-based protocol may be superior to other one-step approaches, e.g., viral transduction of DCs with regard to safety concerns and a more cumbersome process of viral transduction. Most importantly, BGs mixed with oncolysate deliver a variety of neo-TAAs rather than one or several well-defined TAAs. This fact may potentially avoid failure of therapeutic cancer vaccinations due to the emergence of immune escape variants of cancer cells as a result of antigen loss during the immunoediting process (immunoselection) [38, 39].
Here, we showed that human monocyte-derived DCs activated with oncolysate-loaded BGs, prepared by a simple reconstitution of lyophilized BGs in oncolysate, can elicit tumor-specific T cell responses. It is assumed that short incubation of immature DCs with oncolysate-BGs mediates two TAA delivery modes: a proportion of TAAs/oncolysate is delivered within the BG envelopes that are phagocytosed by DCs. The rest of the TAAs/oncolysate (which is not loaded within the BG envelope) is endocytosed by DCs, independent of BG phagocytosis. We presume that simultaneous delivery of target TAAs as proteins within the free (non-loaded) oncolysate together with TAAs loaded inside the BG envelope might result in the release of intact (unprocessed) BG content into the cytoplasm of DCs in addition to antigen processing inside the phagosome–endosome fusion complex. Hence, both MHC class I and MHC class II antigen processing and presentation pathways are engaged, which is crucial for the generation of functionally competent durable effector memory T cell responses [40, 41]. This theory can explain our current results showing that the delivery of exogenous TAAs to human DCs by BGs led to their cross-presentation as confirmed by an abundant proliferation of both CD4+ and CD8+ autologous T cells. Moreover, our data show that the primed CD8+ T cells efficiently recognized and killed target human cancer cells naturally expressing TAAs.
The majority of studies have focused on the development of therapeutic cancer vaccines capable of stimulating cytotoxic CD8+ T cells, which are considered to be the most specific and direct killers of tumor cells [42]. However, the optimal induction of fully functionally competent cytotoxic CD8+ T cells essentially requires the presence of TAA-specific CD4+ T helper cells [43]. Furthermore, direct tumor cell killing properties were also confirmed for CD4+ T cells [44]. Hence, mature clinical-grade DCs should be able to prime and stimulate both CD4+ and CD8+ T cells to trigger balanced anti-tumor immune responses and achieve maximal immune-mediated control of tumor growth [45]. Therefore, it is critical to develop improved TAA-loading approaches capable of cross-presenting exogenous TAAs within both MHC class I and class II molecules. In this regard, BGs emerge as an invaluable tool for optimal DC loading with TAAs since BGs carry highly effective membrane structures, including PAMPs [27], and can stimulate cross-presentation of exogenous, oncolysate-related TAAs by DCs. Indeed, intact LPS affects endosomal acidification of DCs, thereby improving cross-presentation of antigens [46]. Moreover, activation of DCs by IFN-α promotes and strongly enhances cross-presentation of extracellular antigens [47] and, together with BGs, makes the signaling for cross-priming more efficient.
We used IFN-α (with GM-CSF) for both the induction of DC differentiation from monocytes and the induction of their maturation in the presence of oncolysate-loaded BGs. Several recent reports described a critical role of IFN-γ rather than IFN-α for DC activation by various TLR agonists, such as LPS [8, 9, 11, 13]. Despite a lower migratory capacity of LPS/IFN-γ DCs (which can be solved by their intranodal administration), these DCs showed unique immunostimulatory properties and the ability to inhibit the T regulatory cell (Treg)-mediated immunosuppressive effects and the capacity to convert Tregs into effector IFN-γ-producing T cells [15, 48]. In fact, strong immunostimulatory properties, such as the ability to induce Th1-polarized immune responses and shift the T cell phenotype from regulatory to effector, were detected after vaccination of patients with DCs generated in the presence of IFN-α and TLR agonists [49, 50]. In general, type-I IFNs (IFN-α and IFN-β) seem to be required for upregulation of all pathways associated with DC immunogenicity [51]. Furthermore, in our protocol, IFN-α is used for both the induction of DC differentiation and their maturation, hence it avoids the use of additional cytokines, which is more straightforward, cost-effective and standardized for application in a clinical setting.
Our study presents a novel straightforward approach for the ex vivo generation of clinical-grade antigen-loaded mature human DCs. Our findings confirmed that this one-step antigen delivery and maturation-inducing platform generates DCs capable of inducing prominent proliferation of autologous CD4+ and CD8+ T cells that efficiently recognize oncolysate-derived TAAs expressed on the surface of native human cancer cells. Our results provide the opportunity to develop a novel standardized future approach for producing next-generation clinical-grade therapeutic cancer vaccines. The effectiveness of DCs generated by the proposed protocol should be evaluated in clinical trials.
Abbreviations
- APC:
-
Antigen-presenting cell
- BG:
-
Bacterial ghost
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- DC:
-
Dendritic cell
- EMA:
-
European Medicines Agency
- FDA:
-
US Food and Drug Administration
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- IFN:
-
Interferon
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- PAMP:
-
Pathogen-associated molecular pattern
- PBMC:
-
Peripheral blood mononuclear cell
- PHA:
-
Phytohemagglutinin
- PI:
-
Propidium iodide
- poly I:C:
-
Polyinosinic:polycytidylic acid
- PRR:
-
Pattern recognition receptor
- TAA:
-
Tumor-associated antigen
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- WHO/IARC:
-
World Health Organization/International Agency for Research on Cancer
References
Stewart BW, Wild CP (eds) (2014) World cancer report 2014. International agency for research on cancer, Lyon, France
Chen G, Emens LA (2013) Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother 62(2):203–216. doi:10.1007/s00262-012-1388-0
Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12(4):237–251. doi:10.1038/nrc3237
Bhatia A, Kumar Y (2014) Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol 10(1):41–62. doi:10.1586/1744666X.2014.865519
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G (2013) Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39(1):74–88. doi:10.1016/j.immuni.2013.06.014
Nguyen T, Urban J, Kalinski P (2014) Therapeutic cancer vaccines and combination immunotherapies involving vaccination. Immunotargets Ther 3:135–150. doi:10.2147/ITT.S40264
Schijns V, Tartour E, Michalek J, Stathopoulos A, Dobrovolskiene NT, Strioga MM (2014) Immune adjuvants as critical guides directing immunity triggered by therapeutic cancer vaccines. Cytotherapy 16(4):427–439. doi:10.1016/j.jcyt.2013.09.008
Palucka K, Banchereau J (2013) Dendritic-cell-based therapeutic cancer vaccines. Immunity 39(1):38–48. doi:10.1016/j.immuni.2013.07.004
Strioga MM, Felzmann T, Powell DJ Jr, Ostapenko V, Dobrovolskiene NT, Matuskova M, Michalek J, Schijns VE (2013) Therapeutic dendritic cell-based cancer vaccines: the state of the art. Crit Rev Immunol 33(6):489–547. doi:10.1615/CritRevImmunol.2013008033
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422. doi:10.1056/NEJMoa1001294
Butterfield LH (2013) Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front Immunol 4:454. doi:10.3389/fimmu.2013.00454
Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN (2014) Clinical use of dendritic cells for cancer therapy. Lancet Oncol 15(7):e257–e267. doi:10.1016/S1470-2045(13)70585-0
Datta J, Terhune JH, Lowenfeld L, Cintolo JA, Xu S, Roses RE, Czerniecki BJ (2014) Optimizing dendritic cell-based approaches for cancer immunotherapy. Yale J Biol Med 87(4):491–518
Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P (2004) Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res 64(17):5934–5937. doi:10.1158/0008-5472.CAN-04-1261
Lee MKt, Xu S, Fitzpatrick EH, Sharma A, Graves HL, Czerniecki BJ (2013) Inhibition of CD4+ CD25+ regulatory T cell function and conversion into Th1-like effectors by a Toll-like receptor-activated dendritic cell vaccine. PLoS ONE 8(11):e74698. doi:10.1371/journal.pone.0074698
Vopenkova K, Mollova K, Buresova I, Michalek J (2012) Complex evaluation of human monocyte-derived dendritic cells for cancer immunotherapy. J Cell Mol Med 16(11):2827–2837. doi:10.1111/j.1582-4934.2012.01614.x
Sundarasetty BS, Chan L, Darling D, Giunti G, Farzaneh F, Schenck F, Naundorf S, Kuehlcke K, Ruggiero E, Schmidt M, von Kalle C, Rothe M, Hoon DS, Gerasch L, Figueiredo C, Koehl U, Blasczyk R, Gutzmer R, Stripecke R (2015) Lentivirus-induced ‘Smart’ dendritic cells: pharmacodynamics and GMP-compliant production for immunotherapy against TRP2-positive melanoma. Gene Ther 22(9):707–720. doi:10.1038/gt.2015.43
Powell KL, Stephens AS, Ralph SJ (2015) Development of a potent melanoma vaccine capable of stimulating CD8(+) T-cells independently of dendritic cells in a mouse model. Cancer Immunol Immunother 64(7):861–872. doi:10.1007/s00262-015-1695-3
Muhammad A, Champeimont J, Mayr UB, Lubitz W, Kudela P (2012) Bacterial ghosts as carriers of protein subunit and DNA-encoded antigens for vaccine applications. Expert Rev Vaccines 11(1):97–116. doi:10.1586/erv.11.149
Ebensen T, Paukner S, Link C, Kudela P, de Domenico C, Lubitz W, Guzman CA (2004) Bacterial ghosts are an efficient delivery system for DNA vaccines. J Immunol 172(11):6858–6865. doi:10.4049/jimmunol.172.11.6858
Kudela P, Paukner S, Mayr UB, Cholujova D, Schwarczova Z, Sedlak J, Bizik J, Lubitz W (2005) Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J Immunother 28(2):136–143
Kudela P, Schwarczova Z, Sedlak J, Bizik J (2001) Conditioned medium from HeLa cells enhances motility of human monocyte-derived dendritic cells but abrogates their maturation and endocytic activity. Neoplasma 48(5):382–388
Ma Y, Shurin GV, Peiyuan Z, Shurin MR (2013) Dendritic cells in the cancer microenvironment. J Cancer 4(1):36–44. doi:10.7150/jca.5046
Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W (2010) The bacterial ghost platform system: production and applications. Bioeng Bugs 1(5):326–336. doi:10.4161/bbug.1.5.12540
Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH (1997) Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 27(12):3135–3142. doi:10.1002/eji.1830271209
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188(1):21–28. doi:10.4049/jimmunol.1101029
Witte A, Wanner G, Sulzner M, Lubitz W (1992) Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli. Arch Microbiol 157(4):381–388
Eko FO, Mania-Pramanik J, Pais R, Pan Q, Okenu DM, Johnson A, Ibegbu C, He C, He Q, Russell R, Black CM, Igietseme JU (2014) Vibrio cholerae ghosts (VCG) exert immunomodulatory effect on dendritic cells for enhanced antigen presentation and induction of protective immunity. BMC Immunol 15(1):584. doi:10.1186/s12865-014-0056-x
Kudela P, Paukner S, Mayr UB, Cholujova D, Kohl G, Schwarczova Z, Bizik J, Sedlak J, Lubitz W (2008) Effective gene transfer to melanoma cells using bacterial ghosts. Cancer Lett 262(1):54–63. doi:10.1016/j.canlet.2007.11.031
Paukner S, Kudela P, Kohl G, Schlapp T, Friedrichs S, Lubitz W (2005) DNA-loaded bacterial ghosts efficiently mediate reporter gene transfer and expression in macrophages. Mol Ther 11(2):215–223. doi:10.1016/j.ymthe.2004.09.024
de Rosa F, Ridolfi L, Ridolfi R, Gentili G, Valmorri L, Nanni O, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, Soldati V, Cassan S, Riccobon A, Parisi E, Romeo A, Turci L, Guidoboni M (2014) Vaccination with autologous dendritic cells loaded with autologous tumor lysate or homogenate combined with immunomodulating radiotherapy and/or preleukapheresis IFN-alpha in patients with metastatic melanoma: a randomised “proof-of-principle” phase II study. J Transl Med 12:209. doi:10.1186/1479-5876-12-209
Eyrich M, Schreiber SC, Rachor J, Krauss J, Pauwels F, Hain J, Wolfl M, Lutz MB, de Vleeschouwer S, Schlegel PG, Van Gool SW (2014) Development and validation of a fully GMP-compliant production process of autologous, tumor-lysate-pulsed dendritic cells. Cytotherapy 16(7):946–964. doi:10.1016/j.jcyt.2014.02.017
Gonzalez FE, Ortiz C, Reyes M, Dutzan N, Patel V, Pereda C, Gleisner MA, Lopez MN, Gutkind JS, Salazar-Onfray F (2014) Melanoma cell lysate induces CCR7 expression and in vivo migration to draining lymph nodes of therapeutic human dendritic cells. Immunology 142(3):396–405. doi:10.1111/imm.12264
Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, Montone K, Mantia-Smaldone GM, Smith L, Nisenbaum HL, Levine BL, Kalos M, Czerniecki BJ, Torigian DA, Powell DJ Jr, Mick R, Coukos G (2013) A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res 19(17):4801–4815. doi:10.1158/1078-0432.CCR-13-1185
Truxova I, Pokorna K, Kloudova K, Partlova S, Spisek R, Fucikova J (2014) Day 3 Poly (I:C)-activated dendritic cells generated in CellGro for use in cancer immunotherapy trials are fully comparable to standard Day 5 DCs. Immunol Lett 160(1):39–49. doi:10.1016/j.imlet.2014.03.010
Win SJ, McMillan DG, Errington-Mais F, Ward VK, Young SL, Baird MA, Melcher AA (2012) Enhancing the immunogenicity of tumour lysate-loaded dendritic cell vaccines by conjugation to virus-like particles. Br J Cancer 106(1):92–98. doi:10.1038/bjc.2011.538
Schreiber H, Rowley JD, Rowley DA (2011) Targeting mutations predictably. Blood 118(4):830–831. doi:10.1182/blood-2011-06-357541
Lakshminarayanan V, Supekar NT, Wei J, McCurry DB, Dueck AC, Kosiorek HE, Trivedi PP, Bradley JM, Madsen CS, Pathangey LB, Hoelzinger DB, Wolfert MA, Boons GJ, Cohen PA, Gendler SJ (2016) MUC1 vaccines, comprised of glycosylated or non-glycosylated peptides or tumor-derived MUC1, can circumvent immunoediting to control tumor growth in MUC1 transgenic mice. PLoS ONE 11(1):e0145920. doi:10.1371/journal.pone.0145920
Nicholaou T, Chen W, Davis ID, Jackson HM, Dimopoulos N, Barrow C, Browning J, Macgregor D, Williams D, Hopkins W, Maraskovsky E, Venhaus R, Pan L, Hoffman EW, Old LJ, Cebon J (2011) Immunoediting and persistence of antigen-specific immunity in patients who have previously been vaccinated with NY-ESO-1 protein formulated in ISCOMATRIX. Cancer Immunol Immunother 60(11):1625–1637. doi:10.1007/s00262-011-1041-3
Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK (2000) Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med 192(11):1535–1544
Neidhardt-Berard EM, Berard F, Banchereau J, Palucka AK (2004) Dendritic cells loaded with killed breast cancer cells induce differentiation of tumor-specific cytotoxic T lymphocytes. Breast Cancer Res 6(4):R322–R328. doi:10.1186/bcr794
Aguilar LK, Guzik BW, Aguilar-Cordova E (2011) Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem 112(8):1969–1977. doi:10.1002/jcb.23126
Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M (2000) Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol 164(7):3902–3912
Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP (2010) Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 207(3):637–650. doi:10.1084/jem.20091918
Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB, Schadendorf D, Croockewit A, Blokx WA, Van Rossum MM, Kwok WW, Adema GJ, Punt CJ, Figdor CG (2013) Targeting CD4+ T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 73(1):19–29. doi:10.1158/0008-5472.CAN-12-1127
Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I (2003) Activation of lysosomal function during dendritic cell maturation. Science 299(5611):1400–1403. doi:10.1126/science.1080106
Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, Santini SM, Ferrantini M (2012) IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 119(6):1407–1417. doi:10.1182/blood-2011-06-363564
Berk E, Xu S, Czerniecki BJ (2014) Dendritic cells matured in the presence of TLR ligands overcome the immunosuppressive functions of regulatory T cells. Oncoimmunology 3:e27617. doi:10.4161/onci.27617
Brezar V, Ruffin N, Richert L, Surenaud M, Lacabaratz C, Palucka K, Thiebaut R, Banchereau J, Levy Y, Seddiki N (2015) Decreased HIV-specific T-regulatory responses are associated with effective DC-vaccine induced immunity. PLoS Pathog 11(3):e1004752. doi:10.1371/journal.ppat.1004752
Santini SM, Lapenta C, Santodonato L, D’Agostino G, Belardelli F, Ferrantini M (2009) IFN-alpha in the generation of dendritic cells for cancer immunotherapy. Handb Exp Pharmacol 188:295–317. doi:10.1007/978-3-540-71029-5_14
Pantel A, Teixeira A, Haddad E, Wood EG, Steinman RM, Longhi MP (2014) Direct type I IFN but not MDA5/TLR3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly IC stimulation. PLoS Biol 12(1):e1001759. doi:10.1371/journal.pbio.1001759
Acknowledgements
We dedicate this work to all the subjects who voluntarily provided biological material for our studies. This work was supported by BIRD-C GmbH Vienna, Austria (www.bird-c.at) and the International Consortium for Cell Therapy and Immunotherapy (http://www.iccti.eu/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jaroslav Michalek is a founder and CEO of Cellthera, s.r.o., Brno, Czech Republic, and inventor of Patent EP2591798B1 Vaccine for use in tumor immunotherapy. Werner Lubitz is a founder and CEO of BIRD-C GmbH, Vienna, Austria, which has licensed the rights to the Bacterial Ghosts Technology, and inventor and proprietor of Patent EP2591798B1 Vaccine for use in tumor immunotherapy. Pavol Kudela is an employee of BIRD-C GmbH, Vienna, Austria, and inventor of Patent EP2591798B1 Vaccine for use in tumor immunotherapy. All other authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michalek, J., Hezova, R., Turanek-Knötigova, P. et al. Oncolysate-loaded Escherichia coli bacterial ghosts enhance the stimulatory capacity of human dendritic cells. Cancer Immunol Immunother 66, 149–159 (2017). https://doi.org/10.1007/s00262-016-1932-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1932-4