Abstract
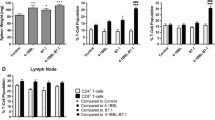
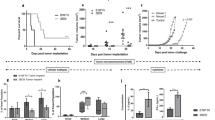
Previously, we combined p19Arf (Cdkn2a, tumor suppressor protein) and interferon beta (IFN-β, immunomodulatory cytokine) gene transfer in order to enhance cell death in a murine model of melanoma. Here, we present evidence of the immune response induced when B16 cells succumbing to death due to treatment with p19Arf and IFN-β are applied in vaccine models. Use of dying cells for prophylactic vaccination was investigated, identifying conditions for tumor-free survival. After combined p19Arf and IFN-β treatment, we observed immune rejection at the vaccine site in immune competent and nude mice with normal NK activity, but not in NOD-SCID and dexamethasone immunosuppressed mice (NK deficient). Combined treatment induced IL-15, ULBP1, FAS/APO1 and KILLER/DR5 expression, providing a mechanism for NK activation. Prophylactic vaccination protected against tumor challenge, where markedly delayed progression and leukocyte infiltration were observed. Analysis of primed lymphocytes revealed secretion of TH1-related cytokines and depletion protocols showed that both CD4+ and CD8+ T lymphocytes are necessary for immune protection. However, application of this prophylactic vaccine where cells were treated either with IFN-β alone or combined with p19Arf conferred similar immune protection and cytokine activation, yet only the combination was associated with increased overall survival. In a therapeutic vaccine protocol, only the combination was associated with reduced tumor progression. Our results indicate that by harnessing cell death in an immunogenic context, our p19Arf and IFN-β combination offers a clear advantage when both genes are included in the vaccine and warrants further development as a novel immunotherapy for melanoma.






Similar content being viewed by others
Abbreviations
- Ad:
-
Adenovirus
- Arf:
-
Alternate reading frame
- CCL:
-
Chemokine (C–C motif) ligand
- CDKN2A:
-
Cyclin-dependent kinase inhibitor 2A
- CXCL:
-
Chemokine (C–X–C motif) ligand
- DEX:
-
Dexamethasone
- EPM-UNIFESP:
-
Escola Paulista de Medicina-Universidade Federal de São Paulo
- FAS/APO1:
-
Fas receptor/apoptosis antigen 1
- FMUSP:
-
Faculdade de Medicina-Universidade de São Paulo
- H60a:
-
Histocompatibility 60a
- ICESP:
-
Instituto do Câncer do Estado de São Paulo
- IU:
-
Infectious units
- KILLER/DR5:
-
KILLER/death receptor 5
- LUC:
-
Luciferase
- MDM2, 4:
-
Murine double minute 2, 4
- MOI:
-
Multiplicity of infection
- NOD-SCID:
-
Non-obese diabetic-severe combined immunodeficiency
- PG:
-
p53-responsive promoter
- PGTxβ:
-
p53-responsive promoter
- Raet 1d, e:
-
Retinoic acid early transcript 1d, e
- RGD:
-
Argenylglycylaspartic acid
- SEM:
-
Standard error of the mean
- TP53:
-
Tumor protein 53, p53
- ULBP1:
-
UL16-binding protein 1
- UNIFESP:
-
Universidade Federal de São Paulo
References
Lu M, Miller P, Lu X (2014) Restoring the tumour suppressive function of p53 as a parallel strategy in melanoma therapy. FEBS Lett 588:2616–2621
Sherr CJ (2006) Divorcing ARF and p53: an unsettled case. Nat Rev Cancer 6:663–673
Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D (1999) Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol 1:20–26
Kaufman HL, Kirkwood JM, Hodi FS et al (2013) The society for immunotherapy of cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol 10:588–598
Thalanayar PM, Agarwala SS, Tarhini AA (2014) Melanoma adjuvant therapy. Chin Clin Oncol 3:26
Diamond MS, Kinder M, Matsushita H et al (2011) Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 208:1989–2003
de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BR (2001) Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol 69:912–920
Bajgelman MC, Strauss BE (2008) Development of an adenoviral vector with robust expression driven by p53. Virology 371:8–13
Merkel CA, Medrano RF, Barauna VG, Strauss BE (2013) Combined p19Arf and interferon-beta gene transfer enhances cell death of B16 melanoma in vitro and in vivo. Cancer Gene Ther 20:317–325
Oba-Shinjo SM, Correa M, Ricca TI et al (2006) Melanocyte transformation associated with substrate adhesion impediment. Neoplasia 8:231–241
Keil D, Luebke RW, Pruett SB (2001) Quantifying the relationship between multiple immunological parameters and host resistance: probing the limits of reductionism. J Immunol 167:4543–4552
Budzynski W, Radzikowski C (1994) Cytotoxic cells in immunodeficient athymic mice. Immunopharmacol Immunotoxicol 16:319–346
Shultz LD, Schweitzer PA, Christianson SW et al (1995) Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154:180–191
Kroemer G, Galluzzi L, Kepp O, Zitvogel L (2013) Immunogenic cell death in cancer therapy. Annu Rev Immunol 31:51–72
Kepp O, Galluzzi L, Martins I et al (2011) Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev 30:61–69
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G (2002) Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 195:327–333
Mocikat R, Braumuller H, Gumy A et al (2003) Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19:561–569
Wong JL, Berk E, Edwards RP, Kalinski P (2013) IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Res 73:4653–4662
Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH (2013) p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 210:2057–2069
Textor S, Fiegler N, Arnold A, Porgador A, Hofmann TG, Cerwenka A (2011) Human NK cells are alerted to induction of p53 in cancer cells by upregulation of the NKG2D ligands ULBP1 and ULBP2. Cancer Res 71:5998–6009
Rodrigues L, Bonorino C (2009) Role of IL-15 and IL-21 in viral immunity: applications for vaccines and therapies. Expert Rev Vaccines 8:167–177
Croce M, Orengo AM, Azzarone B, Ferrini S (2012) Immunotherapeutic applications of IL-15. Immunotherapy 4:957–969
Sutherland CL, Rabinovich B, Chalupny NJ, Brawand P, Miller R, Cosman D (2006) ULBPs, human ligands of the NKG2D receptor, stimulate tumor immunity with enhancement by IL-15. Blood 108:1313–1319
Yoon SR, Kim TD, Choi I (2015) Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med 47:e141
Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M (2012) Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe 11:631–642
Acknowledgments
We are grateful for the support of the laboratories of Drs. José Eduardo Krieger (Universidade de São Paulo-USP) and Roger Chammas (Universidade de São Paulo-USP). We thank Dr. Elaine Guadelupe Rodrigues (Universidade Federal de São Paulo- UNIFESP) for helpful discussions as well as Jonatan Ersching and Dr. Mauricio Martins Rodrigues (Universidade Federal de São Paulo- UNIFESP) for assistance with antibody production. We also thank Camila Morais Melo (Faculdade de Medicina da Universidade de São Paulo-FMUSP) for assistance in the immunochemistry assay. Sao Paulo Research Foundation grants (Strauss, B.E) 13/25167-5, 11/50911-4; fellowships (Medrano, R.F.V) 13/09474-5, (Ribeiro, A.H) 11/10656-5, (Catani, J.P.P) 14/11524-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Medrano, R.F.V., Catani, J.P.P., Ribeiro, A.H. et al. Vaccination using melanoma cells treated with p19arf and interferon beta gene transfer in a mouse model: a novel combination for cancer immunotherapy. Cancer Immunol Immunother 65, 371–382 (2016). https://doi.org/10.1007/s00262-016-1807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1807-8