Abstract
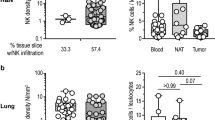
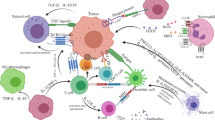
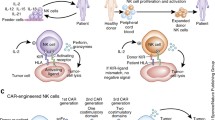
It is well established that natural killer (NK) cells play an important role in the immunity against cancer, while the involvement of other recently identified, NK-related innate lymphoid cells is still poorly defined. In the haploidentical hematopoietic stem cell transplantation for the therapy of high-risk leukemias, NK cells have been shown to exert a key role in killing leukemic blasts residual after conditioning. While the clinical results in the cure of leukemias are excellent, the exploitation of NK cells in the therapy of solid tumors is still limited and unsatisfactory. In solid tumors, NK cell function may be inhibited via different mechanisms, occurring primarily at the tumor site. The cellular interactions in the tumor microenvironment involve tumor cells, stromal cells and resident or recruited leukocytes and may favor tumor evasion from the host’s defenses. In this context, a number of cytokines, growth factors and enzymes synthesized by tumor cells, stromal cells, suppressive/regulatory myeloid and lymphoid cells may substantially impair the function of different tumor-reactive effector cells, including NK cells. The identification and characterization of such mechanisms may offer clues for the development of new immunotherapeutic strategies to restore effective anti-tumor responses. In order to harness NK cell-based immunotherapies, several approaches have been proposed, including reinforcement of NK cell cytotoxicity by means of specific cytokines, antibodies or drugs. These new tools may improve NK cell function and/or increase tumor susceptibility to NK-mediated killing. Hence, the integration of NK-based immunotherapies with conventional anti-tumor therapies may increase chances of successful cancer treatment.

Similar content being viewed by others
Abbreviations
- ACT:
-
Adoptive cell transfer
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- ALL:
-
Acute lymphoblastic leukemia
- AML:
-
Acute myeloid leukemia
- BAT3/BAG6:
-
HLA-B-associated transcript 3/BCL2-associated athanogene 6
- BM:
-
Bone marrow
- CAR:
-
Chimeric antigen receptors
- DNAM-1:
-
DNAX accessory molecule-1
- EBV:
-
Epstein–barr virus
- ELS:
-
Ectopic lymphoid structures
- GvHD:
-
Graft-versus-host disease
- GvL:
-
Graft-versus-leukemia
- HCC:
-
Hepatocellular carcinoma
- HSC:
-
Hematopoietic stem cell
- HSCT:
-
Hematopoietic stem cell transplant
- HSP-90:
-
Heat-shock protein 90
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- ILC:
-
Innate lymphoid cells
- KIRs:
-
Killer cell Ig-like receptors
- LAK:
-
Lymphokine-activated killer
- LSC:
-
Leukemic stem cells
- LTi:
-
Lymphoid tissue inducer
- mAbs:
-
Monoclonal antibodies
- MDSC:
-
Myeloid-derived suppressor cells
- MICA:
-
MHC class I chain-related gene A
- MIF:
-
Migration inhibitory factor
- MSC:
-
Mesenchymal stem cells
- MUC16:
-
Mucin 16
- NIMA:
-
Non-inherited maternal antigen
- NK:
-
Natural killer
- NKG2D:
-
Natural killer group 2, member D
- PB:
-
Peripheral blood
- PCNA:
-
Proliferating cell nuclear antigen
- PE-NK cells:
-
Pleural effusions
- PGE2:
-
Prostaglandin E2
- PS:
-
Phosphatidylserine
- SLOs:
-
Secondary lymphoid organs
- TAF:
-
Tumor-associated fibroblasts
- TAM:
-
Tumor-associated macrophages
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- Tregs:
-
Regulatory T cells
- VPA:
-
Sodium valproate
References
Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331(6013):44–49
Shi FD, Ljunggren HG, La Cava A, Van Kaer L (2011) Organ-specific features of natural killer cells. Nat Rev Immunol 11(10):658–671
Cerwenka A, Lanier LL (2001) Natural killer cells, viruses and cancer. Nat Rev Immunol 1(1):41–49
Fauriat C, Long EO, Ljunggren HG, Bryceson YT (2010) Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115(11):2167–2176
Bryceson YT, March ME, Ljunggren HG, Long EO (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 214:73–91
Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L (1996) Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol 14:619–648
Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L (2001) Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol 19:197–223
Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A (2002) Human natural killer cells: their origin, receptors and function. Eur J Immunol 32(5):1205–1211
Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A (2004) Different checkpoints in human NK-cell activation. Trends Immunol 25(12):670–676
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22(11):633–640
Caligiuri MA (2008) Human natural killer cells. Blood 112(3):461–469
Montaldo E, Del Zotto G, Della Chiesa M, Mingari MC, Moretta A, De Maria A, Moretta L (2013) Human NK cell receptors/markers: a tool to analyze NK cell development, subsets and function. Cytometry A 83(8):702–713
Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, Cipollone G, Navarra G, Mingari MC, Moretta L, Ferlazzo G (2014) CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol 192(8):3805–3815
Carrega P, Ferlazzo G (2012) Natural killer cell distribution and trafficking in human tissues. Front Immunol 3:347
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E (2013) Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 13(2):145–149
Eberl G, Di Santo JP, Vivier E (2015) The brave new world of innate lymphoid cells. Nat Immunol 16(1):1–5
Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjosberg JM, Spits H (2013) Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 14(3):221–229
Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M (2013) Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity 38(4):769–781
Barlow JL, McKenzie AN (2014) Type-2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol 14(5):397–403
Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T, Setoyama H, Imaoka A, Uematsu S, Akira S, Domino SE, Kulig P, Becher B, Renauld JC, Sasakawa C, Umesaki Y, Benno Y, Kiyono H (2014) Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345(6202):1254009
Vacca P, Montaldo E, Croxatto D, Loiacono F, Canegallo F, Venturini PL, Moretta L, Mingari MC (2015) Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol 8(2):254–264
Dyring-Andersen B, Geisler C, Agerbeck C, Lauritsen JP, Gudjonsdottir SD, Skov L, Bonefeld CM (2014) Increased number and frequency of group 3 innate lymphoid cells in nonlesional psoriatic skin. Br J Dermatol 170(3):609–616
Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F (2013) Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 210(5):917–931
Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH (2014) Tertiary lymphoid structures in cancer and beyond. Trends Immunol 35(11):571–580
Pitzalis C, Jones GW, Bombardieri M, Jones SA (2014) Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 14(7):447–462
Burke S, Lakshmikanth T, Colucci F, Carbone E (2010) New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol 31(9):339–345
Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G (2008) Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 112(4):863–875
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, Andre P, Romagne F, Thibault G, Viens P, Birnbaum D, Bertucci F, Moretta A, Olive D (2011) Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 121(9):3609–3622
Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, Sautes-Fridman C, Cremer I (2011) Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 71(16):5412–5422
Konjevic G, Mirjacic Martinovic K, Vuletic A, Jovic V, Jurisic V, Babovic N, Spuzic I (2007) Low expression of CD161 and NKG2D activating NK receptor is associated with impaired NK cell cytotoxicity in metastatic melanoma patients. Clin Exp Metastasis 24(1):1–11
Lee JC, Lee KM, Kim DW, Heo DS (2004) Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 172(12):7335–7340
Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A (2002) Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 99(10):3661–3667
Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, Schirmacher P, Brand K, Grabe N, Falk CS (2011) Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res 17(4):678–689
Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, Moretta L, Mingari MC, Vitale M (2009) Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA 106(49):20847–20852
Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A (2003) Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA 100(7):4120–4125
Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S, Solari N, Gualco M, Queirolo P, Moretta L, Mingari MC (2012) Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 72(6):1407–1415
Marcenaro E, Della Chiesa M, Bellora F, Parolini S, Millo R, Moretta L, Moretta A (2005) IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol 174(7):3992–3998
Ghiringhelli F, Menard C, Martin F, Zitvogel L (2006) The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev 214:229–238
Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L (2005) CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202(8):1075–1085
Sitrin J, Ring A, Garcia KC, Benoist C, Mathis D (2013) Regulatory T cells control NK cells in an insulitic lesion by depriving them of IL-2. J Exp Med 210(6):1153–1165
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F (2009) Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50(3):799–807
Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M, Friess H, Otto G, Heikenwalder M, Protzer U (2013) Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology 57(6):2358–2368
Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G (2012) Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 318(2):154–161
Li T, Yi S, Liu W, Jia C, Wang G, Hua X, Tai Y, Zhang Q, Chen G (2013) Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol 30(3):663
El-Gazzar A, Groh V, Spies T (2013) Immunobiology and conflicting roles of the human NKG2D lymphocyte receptor and its ligands in cancer. J Immunol 191(4):1509–1515
Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, Weller M, Bucala R, Dietl J, Wischhusen J (2008) Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol 180(11):7338–7348
Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, Patankar MS (2010) MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer 9:11
Castriconi R, Dondero A, Bellora F, Moretta L, Castellano A, Locatelli F, Corrias MV, Moretta A, Bottino C (2013) Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol 190(10):5321–5328
Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC, Vitale M (2013) Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol 43(10):2756–2764
Balsamo M, Vermi W, Parodi M, Pietra G, Manzini C, Queirolo P, Lonardi S, Augugliaro R, Moretta A, Facchetti F, Moretta L, Mingari MC, Vitale M (2012) Melanoma cells become resistant to NK-cell-mediated killing when exposed to NK-cell numbers compatible with NK-cell infiltration in the tumor. Eur J Immunol 42(7):1833–1842
Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, McKinnon PJ, Hallek M, Engert A (2007) Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27(6):965–974
Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M, Bessler M, Hansen HP, Tawadros S, Herling M, Kronke M, Hallek M, Pogge von Strandmann E (2013) Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 121(18):3658–3665
Rosental B, Brusilovsky M, Hadad U, Oz D, Appel MY, Afergan F, Yossef R, Rosenberg LA, Aharoni A, Cerwenka A, Campbell KS, Braiman A, Porgador A (2011) Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J Immunol 187(11):5693–5702
Horton NC, Mathew SO, Mathew PA (2013) Novel interaction between proliferating cell nuclear antigen and HLA I on the surface of tumor cells inhibits NK cell function through NKp44. PLoS ONE 8(3):e59552
Cantoni C, Bottino C, Augugliaro R, Morelli L, Marcenaro E, Castriconi R, Vitale M, Pende D, Sivori S, Millo R, Biassoni R, Moretta L, Moretta A (1999) Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J Immunol 29(10):3148–3159
Lankry D, Rovis TL, Jonjic S, Mandelboim O (2013) The interaction between CD300a and phosphatidylserine inhibits tumor cell killing by NK cells. Eur J Immunol 43(8):2151–2161
Sloma I, Jiang X, Eaves AC, Eaves CJ (2010) Insights into the stem cells of chronic myeloid leukemia. Leukemia 24(11):1823–1833
Horton SJ, Huntly BJ (2012) Recent advances in acute myeloid leukemia stem cell biology. Haematologica 97(7):966–974
O’Brien JA, Rizzieri DA (2013) Leukemic stem cells: a review. Cancer Invest 31(4):215–220
Wiseman DH, Greystoke BF, Somervaille TC (2014) The variety of leukemic stem cells in myeloid malignancy. Oncogene 33(24):3091–3098
Costello RT, Fauriat C, Sivori S, Marcenaro E, Olive D (2004) NK cells: innate immunity against hematological malignancies? Trends Immunol 25(6):328–333
Carlsten M, Baumann BC, Simonsson M, Jadersten M, Forsblom AM, Hammarstedt C, Bryceson YT, Ljunggren HG, Hellstrom-Lindberg E, Malmberg KJ (2010) Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34 + blasts in myelodysplastic syndrome. Leukemia 24(9):1607–1616
Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, Pavlu J, Brisley G, de Lavallade H, Sarvaria A, Marin D, Mielke S, Apperley JF, Shpall EJ, Barrett AJ, Rezvani K (2014) Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 99(5):836–847
Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM (2009) The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood 113(11):2394–2401
Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M (2011) Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 96(9):1302–1309
Ayala F, Dewar R, Kieran M, Kalluri R (2009) Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia 23(12):2233–2241
Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM, Qureshi A, Dazzi F, Vyas P, Cerundolo V (2013) Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 122(5):749–758
Duan CW, Shi J, Chen J, Wang B, Yu YH, Qin X, Zhou XC, Cai YJ, Li ZQ, Zhang F, Yin MZ, Tao Y, Mi JQ, Li LH, Enver T, Chen GQ, Hong DL (2014) Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell 25(6):778–793
Tsimberidou AM, Estey E, Wen S, Pierce S, Kantarjian H, Albitar M, Kurzrock R (2008) The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer 113(7):1605–1613
Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR (2010) Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood 116(20):4251–4261
Vitale C, Ambrosini P, Montaldo E, Ballerini F, Moretta L, Mingari MC (2015) IL-1beta-releasing human acute myeloid leukemia blasts modulate natural killer cell differentiation from CD34 + precursors. Haematologica 100(2):e42–e45
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313(23):1485–1492
Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O’Connell M, Khan A, Vlamis V, Vogelzang NJ, Bajorin DF (1995) Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 76(5):824–832
Bordignon C, Carlo-Stella C, Colombo MP, De Vincentiis A, Lanata L, Lemoli RM, Locatelli F, Olivieri A, Rondelli D, Zanon P, Tura S (1999) Cell therapy: achievements and perspectives. Haematologica 84(12):1110–1149
Cheng M, Chen Y, Xiao W, Sun R, Tian Z (2013) NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 10(3):230–252
Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA (2011) Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 17(19):6287–6297
Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB (2005) Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105(8):3051–3057
Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K, Bliss R, Downs LS, Miller JS (2011) A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 13(1):98–107
Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, Perez SA (2010) A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother 59(12):1781–1789
Wennerberg E, Kremer V, Childs R, Lundqvist A (2015) CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother 64(2):225–235
Moustaki A, Argyropoulos KV, Baxevanis CN, Papamichail M, Perez SA (2011) Effect of the simultaneous administration of glucocorticoids and IL-15 on human NK cell phenotype, proliferation and function. Cancer Immunol Immunother 60(12):1683–1695
Vacca P, Martini S, Garelli V, Passalacqua G, Moretta L, Mingari MC (2013) NK cells from malignant pleural effusions are not anergic but produce cytokines and display strong antitumor activity on short-term IL-2 activation. Eur J Immunol 43(2):550–561
Vacca P, Martini S, Mingari MC, Moretta L (2013) NK cells from malignant pleural effusions are potent antitumor effectors: a clue for adoptive immunotherapy? Oncoimmunology 2(4):e23638
Terme M, Fridman WH, Tartour E (2013) NK cells from pleural effusions are potent antitumor effector cells. Eur J Immunol 43(2):331–334
Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A (2012) Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 209(13):2351–2365
Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, Deng W, Ring AM, Fischer S, Garcia KC, Raulet DH (2014) Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest 124(11):4781–4794
Vacchelli E, Galluzzi L, Eggermont A, Galon J, Tartour E, Zitvogel L, Kroemer G (2012) Trial watch: immunostimulatory cytokines. Oncoimmunology 1(4):493–506
Tarhini AA, Millward M, Mainwaring P, Kefford R, Logan T, Pavlick A, Kathman SJ, Laubscher KH, Dar MM, Kirkwood JM (2009) A phase 2, randomized study of SB-485232, rhIL-18, in patients with previously untreated metastatic melanoma. Cancer 115(4):859–868
Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, Goldman CK, Bryant BR, Decker JM, Chen J, Worthy TA, Figg WD Sr, Peer CJ, Sneller MC, Lane HC, Yovandich JL, Creekmore SP, Roederer M, Waldmann TA (2015) Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 33(1):74–82
Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L (2008) Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol 9(5):486–494
Krieg S, Ullrich E (2012) Novel immune modulators used in hematology: impact on NK cells. Front Immunol 3:388
Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM Jr, Blaser BW, Della Chiesa M, Moretta A, Vivier E, Caligiuri MA, Velardi A, Wagtmann N (2009) Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114(13):2667–2677
Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A, Waller E, Ugolini S, Vivier E, Romagne F, Levy R, Blery M, Andre P (2014) Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 123(5):678–686
Oberoi P, Wels WS (2013) Arming NK cells with enhanced antitumor activity: CARs and beyond. Oncoimmunology 2(8):e25220
Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Cunningham-Rundles S, Dupont B, Hodes MZ, Good RA, O’Reilly RJ (1983) Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood 61(2):341–348
Locatelli F, Merli P, Rutella S (2013) At the bedside: innate immunity as an immunotherapy tool for hematological malignancies. J Leukoc Biol 94(6):1141–1157
Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, Iacucci R, Zei T, Martelli MP, Gambelunghe C et al (1994) Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood 84(11):3948–3955
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, Reisner Y, Martelli MF (1998) Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med 339(17):1186–1193
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295(5562):2097–2100
Moretta L, Locatelli F, Pende D, Marcenaro E, Mingari MC, Moretta A (2011) Killer Ig-like receptor-mediated control of natural killer cell alloreactivity in haploidentical hematopoietic stem cell transplantation. Blood 117(3):764–771
Locatelli F, Pende D, Mingari MC, Bertaina A, Falco M, Moretta A, Moretta L (2013) Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: role of alloreactive NK cells. Front Immunol 4:15
Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, Spaggiari GM, Dondero A, Carnemolla B, Reymond N, Mingari MC, Lopez M, Moretta L, Moretta A (2005) PVR (CD155) and nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol 42(4):463–469
Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, Romeo E, Cognet C, Martinetti M, Maccario R, Mingari MC, Vivier E, Moretta L, Locatelli F, Moretta A (2009) Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 113(13):3119–3129
Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R, Bacigalupo A, Mingari MC, Moretta A, Moretta L (2005) Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the poliovirus receptor (CD155) and nectin-2 (CD112). Blood 105(5):2066–2073
Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B (2007) KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol 179(2):854–868
Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, Hsu KC (2012) HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med 367(9):805–816
Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ (2010) Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant 16(4):533–542
Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, Marsh SG, Geraghty D, Spellman S, Haagenson MD, Ladner M, Trachtenberg E, Parham P, Miller JS (2010) Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 116(14):2411–2419
Oevermann L, Michaelis SU, Mezger M, Lang P, Toporski J, Bertaina A, Zecca M, Moretta L, Locatelli F, Handgretinger R (2014) KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood 124(17):2744–2747
Stern M, Ruggeri L, Mancusi A, Bernardo ME, de Angelis C, Bucher C, Locatelli F, Aversa F, Velardi A (2008) Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood 112(7):2990–2995
Ichinohe T, Teshima T, Matsuoka K, Maruya E, Saji H (2005) Fetal-maternal microchimerism: impact on hematopoietic stem cell transplantation. Curr Opin Immunol 17(5):546–552
Dutta P, Burlingham WJ (2009) Tolerance to noninherited maternal antigens in mice and humans. Curr Opin Organ Transplant 14(4):439–447
Handgretinger R (2012) New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol 39(6):664–673
Locatelli F, Moretta F, Brescia L, Merli P (2014) Natural killer cells in the treatment of high-risk acute leukaemia. Semin Immunol 26(2):173–179
Norell H, Moretta A, Silva-Santos B, Moretta L (2013) At the bench: preclinical rationale for exploiting NK cells and gammadelta T lymphocytes for the treatment of high-risk leukemias. J Leukoc Biol 94(6):1123–1139
Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, Meazza R, Loiacono F, Lucarelli B, Bernardo ME, Barbarito G, Pende D, Moretta A, Pistoia V, Moretta L, Locatelli F (2015) gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta +/CD19 + lymphocytes. Blood 125(15):2349–2358
Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, Pende D, Falco M, Handgretinger R, Moretta F, Lucarelli B, Brescia LP, Li Pira G, Testi M, Cancrini C, Kabbara N, Carsetti R, Finocchi A, Moretta A, Moretta L, Locatelli F (2014) HLA-haploidentical stem cell transplantation after removal of alphabeta + T and B cells in children with nonmalignant disorders. Blood 124(5):822–826
Acknowledgments
This work was supported by Grants awarded by Associazione Italiana Ricerca sul Cancro (AIRC): IG 2010 project n. 10225 (to L. Moretta), IG 2014 project n. 15283 (to L. Moretta) and “Special Program Molecular Clinical Oncology 5 × 1000” project n. 9962 (to L. Moretta). This work was also supported by Italian Ministry of Health Grants RF-2010-2316606 (to F. Locatelli, L. Moretta and D. Pende) and RF-2010-2316319 (to D. Pende).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict of interest to disclose.
Additional information
This article is part of the Symposium-in-Writing “Natural killer cells, ageing and cancer”, a series of papers published in Cancer Immunology, Immunotherapy.
Rights and permissions
About this article
Cite this article
Pietra, G., Vitale, C., Pende, D. et al. Human natural killer cells: news in the therapy of solid tumors and high-risk leukemias. Cancer Immunol Immunother 65, 465–476 (2016). https://doi.org/10.1007/s00262-015-1744-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1744-y