Abstract
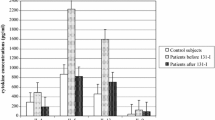
Hashimoto thyroiditis (HT) is the most frequent thyroid autoimmune disease, while papillary thyroid cancer (PTC) is one of the most common endocrine malignancies. A few patients with HT also develop PTC. The aim of this study was to analyze cytokine profiles in patients with PTC accompanied with autoimmune HT in comparison with those in patients with PTC alone or HT alone and healthy subjects. Cytokine levels were determined in supernatants obtained from phytohemagglutinin (PHA)-stimulated whole blood cultures in vitro. The concentrations of selected cytokines: Th1—interferon gamma (IFN-γ); Th2—interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 10 (IL-10) and interleukin 13 (IL-13); Th9—interleukin 9 (IL-9); and Th17—interleukin 17 (IL-17A) were measured using multiplex cytokine detection systems for human Th1/Th2/Th9/Th17/Th22. We found that PTC patients with HT produced significantly higher concentrations of IL-4, IL-6, IL-9, IL-13 and IFN-γ than PTC patients without HT. In conclusion, autoimmune HT affects the cytokine profile of patients with PTC by stimulating secretion of Th1/Th2/Th9 types of cytokines. Th1/Th2 cytokine ratios in PTC patients with associated autoimmune HT indicate a marked shift toward Th2 immunity.



Similar content being viewed by others
Abbreviations
- Abs:
-
Antibodies
- HT:
-
Hashimoto thyroiditis
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- PHA:
-
Phytohemagglutinin
- PTC:
-
Papillary thyroid cancer
- Tg:
-
Thyroglobulin
- Th1:
-
T-helper-1
- Th17:
-
T-helper-17
- Th2:
-
T-helper-2
- Th9:
-
T-helper-9
- TPO:
-
Thyroperoxidase
References
Dayan CM, Daniels GH (1996) Chronic autoimmune thyroiditis. N Engl J Med 335:99–107
Mazziotti G, Sorvillo F, Naclerio C et al (2003) Type-1 response in peripheral CD4+ and CD8+ T cells from patients with Hashimoto’s thyroiditis. Eur J Endocrinol 148:383–388
Figueroa-Vega N, Alfonso-Pérez M, Benedicto I et al (2010) Increased circulating proinflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab 2010(95):953–962
Skapenko A, Niedobitek GU, Kalden JR et al (2004) Generation and regulation of human Th1-biased immune responses in vivo: a critical role for IL-4 and IL-10. J Immunol 172:6427–6434
Marazuela M, García-López MA, Figueroa-Vega N et al (2006) Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab 91:3639–3646
Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167
Riesco-Eizaguirre G, Santisteban P (2007) New insights in thyroid follicular cell biology and its impact in thyroid cancer therapy. Endocr Relat Cancer 14:957–977
Ozgen AG, Karadeniz M, Erdogan M et al (2009) The (-174) G/C polymorphism in the interleukin-6 gene is associated with risk of papillary thyroid carcinoma in Turkish patients. J Endocrinol Invest 32:491–494
Cunha LL, Aj Tincani, Assumpcao LV et al (2011) Interleukin-10 but not interleukin-18 may be associated with the immune response against well-differentiated thyroid cancer. Clinics (Sao Paulo) 66:1203–1208
Simonovic SZ, Mihaljevic O, Majstorovic I et al (2015) Cytokine production in peripheral blood cells of patients with differentiated thyroid cancer: elevated Th2/Th9 production before and reduced Th2 cytokine production after radioactive iodine therapy. Cancer Immunol Immunother 64:75–82
Stassi G, Todaro M, Zerilli M et al (2003) Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res 63:6784–6790
Jankovic B, Le KT, Hershman JM (2013) Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab 98:474–482
Weber F (2014) Lymphocytes and thyroid cancer: more to it than meets the eye? Endocr Relat Cancer 21:C1–C5
Cunha LL, Marcello MA, Ward LS (2014) The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocr Relat Cancer 21:R85–R103
Kashima K, Yokoyama S, Noguchi S et al (1998) Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid 8:197–202
Singh B, Shaha AR, Trivedi H et al (1999) Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact of presentation, management and outcome. Surgery 126:1070–1077
Kim EY, Kim WG, Kim WB et al (2009) Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 71:581–586
Lee JH, Kim Y, Choi JW et al (2013) The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol 168:343–349
Kebebew E, Treseler P, Ituarte P, Clark O (2001) Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg 25:632–637
Cunha LL, Ward LS (2012) Concurrent lymphocytic thyroiditis is associated to less aggressive papillary thyroid carcinomas. Eur Arch Otorhinolaryngol 269:699–700
Schuetz M, Duan H, Wahl K et al (2006) T Lymphocyte cytokine production patterns in Hashimoto patients with elevated calcitonin levels and their relationship to tumor initiation. Anticancer Res 26:4591–4596
Nikiforov YE (2012) Thyroid tumors: classification, staging, and general considerations. In: Nikiforov YE, Biddinger PW, Thompson LDR (eds) Diagnostic pathology and molecular genetics of the thyroid, 2nd edn. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, pp 108–119
Budhu A, Wang XW (2006) The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 80:1197–1213
Bodelon C, Polley MY, Kemp TJ et al (2013) Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann Oncol 24:2073–2079
Ellyard JI, Simson L, Parish CR (2007) Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens 70:1–11
Mattes J, Hulett M, Xie W et al (2003) Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med 197:387–393
Roy M, Chen H, Sippel RS (2013) Current understanding and management of medullary thyroid cancer. Oncologist 18:1093–1100
Schroder K, Hertzog PJ, Ravasi T et al (2004) Interferon-Υ: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189
Del Prete GF, Tiri A, Mariotti S et al (1987) Enhanced production of gamma-interferon by thyroid-derived T cell clones from patients with Hashimoto’s thyroiditis. Clin Exp Immunol 69:323–331
Zaidi MR, Merlino G (2011) The two faces of interferon-Υ in cancer. Clin Cancer Res 17:6118–6124
Miller CH, Maher SG, Young HA (2009) Clinical use of interferon-gamma. Ann N Y Acad Sci 1182:69–79
Hossain MS, Bhimani C, Zhengjia C et al (2011) Profiling counter-regulatory and cytotoxic immune pathways with cellular biomarkers in thyroid cancer patients. [abstract]. In: Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 Apr 2–6; Orlando, FL. Philadelphia (PA): AACR; Cancer Res 71(8 Suppl):Abstract nr 5525. doi:10.1158/1538-7445.AM2011-5525
Kang S, Tanaka T, Kishimoto T (2015) Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol 27:21–29
Kimura A, Kishimoto T (2010) IL-6: regulator of Treg/Th17 balance. Eur J Immunol 40:1830–1835
Baki M, Akman FE, Vural P et al (2012) The combination of interleukin-10 -1082 and tumor necrosis factor α -308 or interleukin-6 -174 genes polymorphisms suggests an association with susceptibility to Hashimoto’s thyroiditis. Int Immunopharmacol 12:543–546
Weetman AP, Bright-Thomas R, Freeman M (1990) Regulation of interleukin-6 release by human thyrocytes. J Endocrinol 127:357–361
Ajjan RA, Watson PF, Weetman AP (1996) Cytokines and thyroid function. Adv Neuroimmunol 6:359–386
Ruggeri RM, Sciacchitano S, Vitale A et al (2009) Serum hepatocyte growth factor (HGF) is increased in Hashimoto’s thyroiditis whether or not it is associated with nodular goiter as compared with healthy non-goitrous individuals. J Endocrinol Invest 32:465–469
Sieminska L, Wojciechowska C, Kos-Kudla B et al (2010) Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol 61:112–116
Sehgal PB (1990) Interleukin 6 in infection and cancer. Proc Soc Exp Biol Med 195:183–191
Kura Y, De Velasco MA, Kobayashi Y, et al (2013) Interleukin-6 (IL-6) as a therapeutic target in prostate cancer. [abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC. Philadelphia (PA): AACR; Cancer Res 73(8 Suppl):Abstract nr 1226. doi:10.1158/1538-7445.AM2013-1226
Zhang Y, Yan W, Collins MA et al (2013) Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res 73:6359–6374
Ujiie H, Tomida M, Akiyama H et al (2012) Serum hepatocyte growth factor and interleukin-6 are effective prognostic markers for non-small cell lung cancer. Anticancer Res 32:3251–3258
Huang BY, Hseuh C, Chao TC et al (2011) Well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol 22:144–149
Ng TH, Britton GJ, Hill EV et al (2013) Regulation of adaptive immunity; the role of interleukin-10. Front Immunol 4:129–140
Smilek DE, Ehlers MR, Nepom GT (2014) Restoring the balance: immunotherapeutic combinations for autoimmune disease. Dis Model Mech 7:503–513
de Waal-Malefyt R, Yssel H, de Vries JE (1993) Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol 150:4754–4765
Cyktor JC, Turner J (2011) Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect Immunol 79:2964–2973
Franks AL, Slansky JE (2012) Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 32:1119–1136
Luheshi N, Davies GC, Poon E et al (2013) Th1 and Th2 cytokines determine how CD40 activation changes human macrophage function in vitro. [abstract]. In: Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC. Philadelphia (PA): AACR; Cancer Res 73(8 Suppl):Abstract nr 1542. doi:10.1158/1538-7445.AM2013-1542
Becker JC, Andersen MH, Schrama D et al (2013) Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother 62:1137–1148
Atsumi T, Singh R, Sabharwal L et al (2014) Inflammation amplifier, a new paradigm in cancer biology. Cancer Res 74:8–14
Murray JI, West NR, Murphy LC et al (2014) Intratumoral inflammation and endocrine resistance in breast cancer. Endocr Relat Cancer 22:R51–R67
Acknowledgments
The authors would like to thank the anonymous reviewers whose remarks and suggestions have highly improved the final version of our paper. The study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant Nos. III41010 and ON175069) and Faculty of Medical Sciences University of Kragujevac, Serbia (JP 06-12).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zivancevic-Simonovic, S., Mihaljevic, O., Majstorovic, I. et al. Cytokine production in patients with papillary thyroid cancer and associated autoimmune Hashimoto thyroiditis. Cancer Immunol Immunother 64, 1011–1019 (2015). https://doi.org/10.1007/s00262-015-1705-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1705-5