Abstract
Background
We conducted a phase I dose escalation study to evaluate the safety and immunologic response to peptide immunomodulatory vaccines GL-0810 (HPV16) and GL-0817 (MAGE-A3) in HPV16 and MAGE-A3-positive RM–SCCHN patients, respectively.
Methods
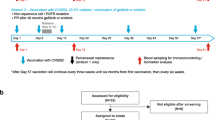
Three dose levels (500, 1,000, and 1,500 µg) of GL-0810 or GL-0817 with adjuvants Montanide (1.2 ml) and GM-CSF (100 µg/m2) were administered subcutaneously q2 weeks for a total of four vaccinations in HPV16 and MAGE-A3-positive RM–SCCHN patients, respectively.
Results
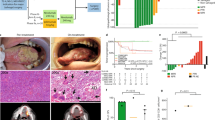
Nine and seven patients were enrolled in the HPV16 and MAGE-A3 cohorts, respectively. No dose-limiting toxicities were observed, and toxicity was predominantly local and grade 1 (erythema, pain, and itching at the injection site). In those patients who received all four vaccinations, 80 % (4/5) of the HPV16 cohort and 67 % (4/6) of the MAGE-A3 cohort developed antigen-specific T cell and antibody responses to the vaccine. Significant concordance between T cell and antibody responses was observed for both groups. No clear dose–response correlation was seen. All patients progressed by RECIST at first repeat imaging, except for one patient in the MAGE-A3 500 µg cohort who had stable disease for 10.5 months. The median PFS and OS for the MAGE-A3 cohorts were 79 and 183 days, respectively, and for the HPV16 cohort 80 and 196 days, respectively.
Conclusions
GL-0810 and GL-0817 were well tolerated in patients with RM–SCCHN with T cell and antibody responses observed in the majority of patients who received all four vaccinations.



Similar content being viewed by others
Abbreviations
- B7H1:
-
B7 homolog 1
- CNS:
-
Central nervous system
- CR:
-
Complete response
- CTCAE:
-
Common toxicity criteria for adverse events
- DLT:
-
Dose-limiting toxicity
- ECOG:
-
Eastern Cooperative Oncology Group
- ELISA:
-
Enzyme-linked immunosorbent assay
- ELISPOT:
-
Enzyme-linked immunospot
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- HPV16:
-
Human papillomavirus 16
- IFN-γ:
-
Interferon gamma
- IL-10:
-
Interleukin 10
- MAGE:
-
Melanoma antigen E
- MDSC:
-
Myeloid derived suppressor cells
- ml:
-
Milliliter
- m2:
-
Meter squared
- MTD:
-
Maximum tolerated dose
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PD-1:
-
Programmed death 1
- PD-L1:
-
Programmed death-ligand 1
- PET/CT:
-
Positron emission tomography–computed tomography
- PGE2:
-
Prostaglandin E2
- PR:
-
Partial response
- RECIST:
-
Response evaluation criteria in solid tumors
- R/M:
-
Recurrent/metastatic
- SCCHN:
-
Squamous cell carcinoma of the head and neck
- SD:
-
Stable disease
- TAP:
-
Transporter associated with antigen processing
- TGF-β:
-
Transforming growth factor beta
- Tregs:
-
Regulatory T cells
- UMGCC:
-
University of Maryland Greenebaum Cancer Center
- μg:
-
Microgram
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. doi:10.3322/caac.20107
McDonald MW, Lawson J, Garg MK et al (2011) ACR appropriateness criteria retreatment of recurrent head and neck cancer after prior definitive radiation expert panel on radiation oncology-head and neck cancer. Int J Radiat Oncol Biol Phys 80:1292–1298. doi:10.1016/j.ijrobp.2011.02.014
Vermorken JB, Specenier P (2010) Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol 21 Suppl 7: vii252–261. doi 10.1093/annonc/mdq453
Gillison ML, Koch WM, Capone RB et al (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92:709–720. doi:10.1093/jnci/92.9.709
Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14:467–475. doi:10.1007/s12105-010-0171-9
Munoz N, Bosch FX, de Sanjose S et al (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348:518–527. doi:10.1056/NEJMoa021641
Kenter GG, Welters MJ, Valentijn AR et al (2008) Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 14:169–177. doi:10.1158/1078-0432.CCR-07-1881
Ressing ME, Sette A, Brandt RM et al (1995) Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol 154:5934–5943
van der Burg SH, Ressing ME, Kwappenberg KM et al (2001) Natural T-helper immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int J Cancer 91:612–618. doi:10.1002/1097-0215
Cuffel C, Rivals JP, Zaugg Y et al (2011) Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. Int J Cancer 128:2625–2634. doi:10.1002/ijc.25607
Figueiredo DL, Mamede RC, Spagnoli GC et al (2011) High expression of cancer testis antigens MAGE-A, MAGE-C1/CT7, MAGE-C2/CT10, NY-ESO-1, and gage in advanced squamous cell carcinoma of the larynx. Head Neck 33:702–707. doi:10.1002/hed.21522
Jungbluth AA, Ely S, DiLiberto M et al (2005) The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood 106:167–174. doi:10.1182/blood-2004-12-4931
Condomines M, Hose D, Raynaud P et al (2007) Cancer/testis genes in multiple myeloma: expression patterns and prognosis value determined by microarray analysis. J Immunol 178:3307–3315. doi:10.4049/jimmunol.178.5.3307
van der Bruggen P, Traversari C, Chomez P et al (1991) A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254:1643–1647. doi:10.1126/science.1840703
Kobayashi H, Song Y, Hoon DS et al (2001) Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res 61:4773–4778
Lu J, Wettstein PJ, Higashimoto Y et al (2001) TAP-independent presentation of CTL epitopes by Trojan antigens. J Immunol 166:7063–7071. doi:10.4049/jimmunol.166.12.7063
Lu J, Higashimoto Y, Appella E, Celis E (2004) Multiepitope Trojan antigen peptide vaccines for the induction of antitumor CTL and Th immune responses. J Immunol 172:4575–4582. doi:10.4049/jimmunol.172.7.4575
Voskens CJ, Sewell D, Hertzano R et al (2012) Induction of MAGE-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck 34:1734–1746. doi:10.1002/hed.22004
Fruh K, Yang Y (1999) Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol 11:76–81. doi:10.1016/S0952-7915(99)80014-4
Cheever MA, Chen W (1997) Therapy with cultured T cells: principles revisited. Immunol Rev 157:177–194. doi:10.1111/j.1600-065X.1997.tb00982.x
Gao FG, Khammanivong V, Liu WJ et al (2002) Antigen-specific CD4 + T-cell help is required to activate a memory CD8 + T cell to a fully functional tumor killer cell. Cancer Res 62:6438–6441
Voskens CJ, Strome SE, Sewell DA (2009) Synthetic peptide-based cancer vaccines: lessons learned and hurdles to overcome. Curr Mol Med 9:683–693
Pichichero ME (2008) Improving vaccine delivery using novel adjuvant systems. Hum Vaccin 4:262–270. doi:10.4161/hv.4.4.5742
Kreimer AR, Johansson M, Waterboer T et al (2013) Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 31:2708–2715. doi:10.1200/JCO.2012.47.2738
Kruit WH, van Ojik HH, Brichard VG et al (2005) Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer 117:596–604. doi:10.1002/ijc.21264
Klebanoff CA, Acquavella N, Yu Z, Restifo NP (2011) Therapeutic cancer vaccines: are we there yet? Immunol Rev 239:27–44. doi:10.1111/j.1600-065X.2010.00979.x
Atanackovic D, Altorki NK, Stockert E et al (2004) Vaccine-induced CD4 + T cell responses to MAGE-3 protein in lung cancer patients. J Immunol 172:3289–3296. doi:10.4049/jimmunol.172.5.3289
Vansteenkiste J, Zielinski M, Linder A et al (2013) Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 31:2396–2403. doi:10.1200/JCO.2012.43.7103
Marchand M, Punt CJ, Aamdal S et al (2003) Immunisation of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: a clinical report. Eur J Cancer 39:70–77. doi:10.1016/S0959-8049(02)00479-3
Kruit WH, Suciu S, Dreno B et al (2013) Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol 31:2413–2420. doi:10.1200/JCO.2012.43.7111
Brinkman JA, Hughes SH, Stone P et al (2007) Therapeutic vaccination for HPV induced cervical cancers. Dis Markers 23:337–352. doi:10.1155/2007/245146
Allen CT, Judd NP, Bui JD, Uppaluri R (2012) The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope 122:144–157. doi:10.1002/lary.21913
Young MR (2006) Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck 28:462–470. doi:10.1002/hed.20331
Lyford-Pike S, Peng S, Young GD et al (2013) Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 73:1733–1741. doi:10.1158/0008-5472.CAN-12-2384
Strome SE, Dong H, Tamura H et al (2003) B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res 63:6501–6505
Ukpo OC, Thorstad WL, Lewis JS Jr (2012) B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol 7:113–121. doi:10.1007/s12105-012-0406-z
Li W, Deng XM, Wang CX et al (2010) Down-regulation of HLA class I antigen in human papillomavirus type 16 E7 expressing HaCaT cells: correlate with TAP-1 expression. Int J Gynecol Cancer 20:227–232. doi:10.1111/IGC.0b013e3181cceec5
Campo MS, Graham SV, Cortese MS et al (2010) HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 407:137–142. doi:10.1016/j.virol.2010.07.044
Schott AK, Pries R, Wollenberg B (2010) Permanent up-regulation of regulatory T-lymphocytes in patients with head and neck cancer. Int J Mol Med 26:67–75. doi:10.3892/ijmm_00000436
Strauss L, Bergmann C, Szczepanski M et al (2007) A unique subset of CD4 + CD25highFoxp3 + T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res 13:4345–4354. doi:10.1158/1078-0432.CCR-07-0472
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22:275–281. doi:10.1016/j.semcancer.2012.01.011
Herbst RS, Arquette M, Shin DM et al (2005) Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol 23:5578–5587. doi:10.1200/JCO.2005.07.120
Zenda S, Onozawa Y, Boku N et al (2007) Single-agent docetaxel in patients with platinum-refractory metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN). Jpn J Clin Oncol 37:477–481. doi:10.1093/jjco/hym059
Le DT, Jaffee EM (2012) Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res 72:3439–3444. doi:10.1158/0008-5472.CAN-11-3912
Lutsiak ME, Semnani RT, De Pascalis R et al (2005) Inhibition of CD4(+)25 + T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105:2862–2868. doi:10.1182/blood-2004-06-2410
Conflict of interest
Dr. Strome is a co-founder and stockholder of Gliknik Inc., a biotechnology company and licensee of the Trojan Peptide Vaccines. Dr. Strome also receives royalties from the Mayo Clinic College of Medicine for intellectual property related to B7-H1 (PD-L1). All other authors have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Scott E. Strome and Martin J. Edelman have share senior authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zandberg, D.P., Rollins, S., Goloubeva, O. et al. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunol Immunother 64, 367–379 (2015). https://doi.org/10.1007/s00262-014-1640-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1640-x