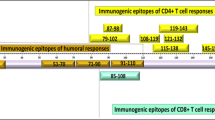
Abstract
Disseminated or relapsed Ewing sarcoma (EwS) has remained fatal in the majority of patients. A promising approach to preventing relapse after conventional therapy is to establish tumor antigen-specific immune control. Efficient and specific T cell memory against the tumor depends on the expansion of rare T cells with native specificity against target antigens overexpressed by the tumor. Candidate antigens in EwS include six-transmembrane epithelial antigen of the prostate-1 (STEAP1), and the human cancer/testis antigens X-antigen family member 1 (XAGE1) and preferentially expressed antigen in melanoma (PRAME). Here, we screened normal donors and EwS patients for the presence of circulating T cells reactive with overlapping peptide libraries of these antigens by IFN-γ Elispot analysis. The majority of 22 healthy donors lacked detectable memory T cell responses against STEAP1, XAGE1 and PRAME. Moreover, ex vivo detection of T cells specific for these antigens in both blood and bone marrow were limited to a minority of EwS patients and required nonspecific T cell prestimulation. Cytotoxic T cells specific for the tumor-associated antigens were efficiently and reliably generated by in vitro priming using professional antigen-presenting cells and optimized cytokine stimulation; however, these T cells failed to interact with native antigen processed by target cells and with EwS cells expressing the antigen. We conclude that EwS-associated antigens fail to induce efficient T cell receptor (TCR)-mediated antitumor immune responses even under optimized conditions. Strategies based on TCR engineering could provide a more effective means to manipulating T cell immunity toward targeted elimination of tumor cells.




Similar content being viewed by others
Abbreviations
- aAPC:
-
Artificial antigen-presenting cell
- APC:
-
Antigen-presenting cell
- BM:
-
Bone marrow
- CTL:
-
Cytotoxic T lymphocyte
- DC:
-
Dendritic cell
- EwS:
-
Ewing sarcoma
- HLA:
-
Human leukocyte antigen
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- MC:
-
Mononuclear cell
- MHC:
-
Major histocompatibility complex
- PB:
-
Peripheral blood
- PD-1:
-
Programmed cell death 1
- PepMix:
-
Peptide mixture
- PRAME:
-
Preferentially expressed antigen in melanoma
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- STEAP1:
-
Six-transmembrane epithelial antigen of the prostate-1
- TCR:
-
T cell receptor
- Treg cell:
-
T cell with regulatory phenotype
- VZV-IE62:
-
Varicella-zoster virus immediate antigen 62
- XAGE1:
-
X-antigen family member 1
References
Stahl M, Ranft A, Paulussen M et al (2011) Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Ped Blood Cancer 57:549–553
Paulussen M, Ahrens S, Burdach S et al (1998) Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European intergroup cooperative Ewing sarcoma studies. Ann Oncol 9:275–281
Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM (2000) Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 6:1018–1023
Montagna D, Maccario R, Locatelli F et al (2006) Emergence of antitumor cytolytic T cells is associated with maintenance of hematologic remission in children with acute myeloid leukemia. Blood 108:3843–3850
Muller-Berghaus J, Ehlert K, Ugurel S, Umansky V, Bucur M, Schirrmacher V, Beckhove P, Schadendorf D (2006) Melanoma-reactive T cells in the bone marrow of melanoma patients: association with disease stage and disease duration. Cancer Res 66:5997–6001
Weide B, Zelba H, Derhovanessian E et al (2012) Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol 30:1835–1841
Feuerer M, Beckhove P, Bai L et al (2001) Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med 7:452–458
Schmitz-Winnenthal FH, Volk C, Z’Graggen K et al (2005) High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res 65:10079–10087
Robbins PF, Morgan RA, Feldman SA et al (2011) Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29:917–924
Bollard CM, Gottschalk S, Leen AM et al (2007) Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood 110:2838–2845
Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R (2006) Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol 24:5060–5069
Mackall CL, Rhee EH, Read EJ et al (2008) A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin Cancer Res 14:4850–4858
Evans CH, Liu F, Porter RM et al (2012) EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors. Clin Cancer Res 18:5341–5351
Hubert RS, Vivanco I, Chen E et al (1999) STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA 96:14523–14528
Staege MS, Hutter C, Neumann I et al (2004) DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res 64:8213–8221
Cheung IY, Feng Y, Danis K, Shukla N, Meyers P, Ladanyi M, Cheung NK (2007) Novel markers of subclinical disease for Ewing family tumors from gene expression profiling. Clin Cancer Res 13:6978–6983
Rodeberg DA, Nuss RA, Elsawa SF, Celis E (2005) Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes. Clin Cancer Res 11:4545–4552
Liu XF, Helman LJ, Yeung C, Bera TK, Lee B, Pastan I (2000) XAGE-1, a new gene that is frequently expressed in Ewing’s sarcoma. Cancer Res 60:4752–4755
Mahlendorf DE, Staege MS (2013) Characterization of Ewing sarcoma associated cancer/testis antigens. Cancer Biol Ther 14:254–261
Ikeda H, Lethe B, Lehmann F et al (1997) Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 6:199–208
Quintarelli C, Dotti G, De Angelis B et al (2008) Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood 112:1876–1885
Weber G, Gerdemann U, Caruana I et al (2013) Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia 27:1538–1547
Alves PMS, Faure O, Graff-Dubois S et al (2006) STEAP, a prostate tumor antigen, is a target of human CD8(+) T cells. Cancer Immunol Immunother 55:1515–1523
Machlenkin A, Paz A, Haim EB et al (2005) Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res 65:6435–6442
Altvater B, Pscherer S, Landmeier S, Kailayangiri S, Savoldo B, Juergens H, Rossig C (2012) Activated human gammadelta T cells induce peptide-specific CD8+ T-cell responses to tumor-associated self-antigens. Cancer Immunol Immunother 61:385–396
Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, Wang C, Davis MM (2003) Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest 111:639–647
Grunewald TG, Ranft A, Esposito I et al (2012) High STEAP1 expression is associated with improved outcome of Ewing’s sarcoma patients. Ann Oncol 23:2185–2190
Grunewald TG, Diebold I, Esposito I et al (2012) STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol Cancer Res 10:52–65
Rezvani K, Grube M, Brenchley JM et al (2003) Functional leukemia-associated antigen-specific memory CD8(+) T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood 102:2892–2900
Rezvani K, Yong AS, Tawab A et al (2009) Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood 113:2245–2255
Scheibenbogen C, Letsch A, Thiel E, Schmittel A, Mailaender V, Baerwolf S, Nagorsen D, Keilholz U (2002) CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood 100:2132–2137
Melenhorst JJ, Scheinberg P, Chattopadhyay PK et al (2009) High avidity myeloid leukemia-associated antigen-specific CD8+ T cells preferentially reside in the bone marrow. Blood 113:2238–2244
Coughlin CM, Fleming MD, Carroll RG et al (2006) Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol 24:5725–5734
Sommerfeldt N, Schutz F, Sohn C, Forster J, Schirrmacher V, Beckhove P (2006) The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res 66:8258–8265
De Angulo G, Hernandez M, Morales-Arias J, Herzog CE, Anderson P, Wolff J, Kleinerman ES (2007) Early lymphocyte recovery as a prognostic indicator for high-risk Ewing sarcoma. J Pediatr Hematol Oncol 29:48–52
Zhang H, Merchant MS, Chua KS et al (2003) Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biol Ther 2:579–586
Beckhove P, Feuerer M, Dolenc M et al (2004) Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest 114:67–76
Khazaie K, Prifti S, Beckhove P, Griesbach A, Russell S, Collins M, Schirrmacher V (1994) Persistence of dormant tumor cells in the bone marrow of tumor cell-vaccinated mice correlates with long-term immunological protection. Proc Natl Acad Sci U S A 91:7430–7434
Feuerer M, Rocha M, Bai L, Umansky V, Solomayer EF, Bastert G, Diel IJ, Schirrmacher V (2001) Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer 92:96–105
Feuerer M, Beckhove P, Garbi N et al (2003) Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med 9:1151–1157
Mazo IB, Honczarenko M, Leung H et al (2005) Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity 22:259–270
Zhang X, Dong H, Lin W et al (2006) Human bone marrow: a reservoir for “enhanced effector memory” CD8+ T cells with potent recall function. J Immunol 177:6730–6737
Berghuis D, de Hooge AS, Santos SJ et al (2009) Reduced human leukocyte antigen expression in advanced-stage Ewing sarcoma: implications for immune recognition. J Pathol 218:222–231
Brinkrolf P, Landmeier S, Altvater B et al (2009) A high proportion of bone marrow T cells with regulatory phenotype (CD4 + CD25hiFoxP3 +) in Ewing sarcoma patients is associated with metastatic disease. Int J Cancer 125:879–886
Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, Mackall CL (2013) Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 122:1105–1113
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA (2009) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114:1537–1544
Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF (2009) Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 114:1528–1536
Zhang L, Gajewski TF, Kline J (2009) PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 114:1545–1552
Zhou Q, Munger ME, Highfill SL et al (2010) Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood 116:2484–2493
Gerdemann U, Katari U, Christin AS et al (2011) Cytotoxic T lymphocytes simultaneously targeting multiple tumor-associated antigens to treat EBV negative lymphoma. Mol Ther 19:2258–2268
Griffioen M, Kessler JH, Borghi M et al (2006) Detection and functional analysis of CD8(+) T cells specific for PRAME: a target for T-cell therapy. Clin Cancer Res 12:3130–3136
Quintarelli C, Dotti G, Hasan ST et al (2011) High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 117:3353–3362
Weber G, Karbach J, Kuci S et al (2009) WT1 peptide-specific T cells generated from peripheral blood of healthy donors: possible implications for adoptive immunotherapy after allogeneic stem cell transplantation. Leukemia 23:1634–1642
Thiel U, Pirson S, Muller-Spahn C, Conrad H, Busch DH, Bernhard H, Burdach S, Richter GH (2011) Specific recognition and inhibition of Ewing tumour growth by antigen-specific allo-restricted cytotoxic T cells. Br J Cancer 104:948–956
Sadovnikova E, Stauss HJ (1996) Peptide-specific cytotoxic T lymphocytes restricted by nonself major histocompatibility complex class I molecules: reagents for tumor immunotherapy. Proc Natl Acad Sci U S A 93:13114–13118
Amir AL, van der Steen DM, van Loenen MM et al (2011) PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin Cancer Res 17:5615–5625
Linette GP, Stadtmauer EA, Maus MV et al (2013) Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122:863–871
Morgan RA, Chinnasamy N, Abate-Daga D et al (2013) Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36:133–151
Parkhurst MR, Yang JC, Langan RC et al (2011) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19:620–626
Spranger S, Jeremias I, Wilde S et al (2012) TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood 119:3440–3449
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725–733
Acknowledgements
This work was supported by pilot funding by the University of Muenster Faculty of Medicine “Innovative Medizinische Forschung (IMF)” program (to Christiane Chen) and by grant Nr. 2010.016.1 from Wilhelm Sander-Stiftung (to Claudia Rossig).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Approval for using tumor, BM and/or PB samples from patients and healthy donors was obtained from the University of Muenster Ethical Board, and informed consent was obtained in accordance with the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Altvater, B., Kailayangiri, S., Theimann, N. et al. Common Ewing sarcoma-associated antigens fail to induce natural T cell responses in both patients and healthy individuals. Cancer Immunol Immunother 63, 1047–1060 (2014). https://doi.org/10.1007/s00262-014-1574-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1574-3