Abstract
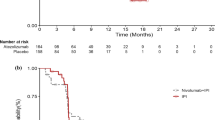
Late divergence of survival curves of treated patients and controls is commonly seen in successful cancer immunotherapy trials. Although late survival curve divergence may be caused by a delayed action of therapy, it may also be related to early effects of the treatment. We suggest that late survival divergence most often reflects a specific benefit of therapy for patients who suffer from a comparatively slow progression of disease. The occurrence of delayed survival curve divergence has important implications for the statistical analysis of immunotherapy trials. Thus, it leads to non-proportional hazard ratios that make commonly used statistical tests, e.g., the logrank test, suboptimal. It is therefore suggested that the statistical analysis of immunotherapy trials primarily should be based on a test that compares the survival curves at or after a prespecified, fixed, late time point.
Similar content being viewed by others
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. doi:10.1056/NEJMoa1003466
Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR (2010) Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28(7):1099–1105. doi:10.1200/jco.2009.25.0597
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM (2006) Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 24(19):3089–3094. doi:10.1200/jco.2005.04.5252
Sosman JA, Unger JM, Liu PY, Flaherty LE, Park MS, Kempf RA, Thompson JA, Terasaki PI, Sondak VK (2002) Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: impact of HLA class I antigen expression on outcome. J Clin Oncol 20(8):2067–2075
Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, Yuh L, Gupta R, Srivastava PK (2008) Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol 26(6):955–962. doi:10.1200/jco.2007.11.9941
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbe C (2010) Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11(2):155–164. doi:10.1016/s1470-2045(09)70334-1
Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, Mulders P, Zielinski H, Hoos A, Teofilovici F, Isakov L, Flanigan R, Figlin R, Gupta R, Escudier B (2008) An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet 372(9633):145–154. doi:10.1016/s0140-6736(08)60697-2
Stadler R, Luger T, Bieber T, Kohler U, Linse R, Technau K, Schubert R, Schroth K, Vakilzadeh F, Volkenandt M, Gollnick H, Von Eick H, Thoren F, Strannegard O (2006) Long-term survival benefit after adjuvant treatment of cutaneous melanoma with dacarbazine and low dose natural interferon alpha: A controlled, randomised multicentre trial. Acta Oncol 45(4):389–399
Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A (2007) Lessons from randomized phase III studies with active cancer immunotherapies—outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine 25(Suppl 2):B97–B109. doi:10.1016/j.vaccine.2007.06.067
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J (2010) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102(18):1388–1397. doi:10.1093/jnci/djq310
Hansson J (2006) Adjuvant therapy of cutaneous melanoma—current status. Acta Oncol 45(4):369–372. doi:10.1080/02841860600768895
Hoos A, Britten CM, Huber C, O’Donnell-Tormey J (2011) A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol 29(10):867–870. doi:10.1038/nbt.2000
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine 364(26):2507–2516. doi:10.1056/NEJMoa1103782
Thoren FB, Strannegard O (2011) Adjuvant interferon: extended follow-up times needed? Lancet Oncol 12(5):419
Berd D, Sato T, Cohn H, Maguire HC Jr, Mastrangelo MJ (2001) Treatment of metastatic melanoma with autologous, hapten-modified melanoma vaccine: regression of pulmonary metastases. Int J Cancer [Journal international du cancer] 94(4):531–539
Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G (2008) Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 105(8):3005–3010. doi:10.1073/pnas.0712237105
Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbe C (2013) Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. doi:10.1093/annonc/mdt161
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422. doi:10.1056/NEJMoa1001294
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3(11):991–998
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29:235–271. doi:10.1146/annurev-immunol-031210-101324
Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ (2012) CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 18(7):2039–2047. doi:10.1158/1078-0432.CCR-11-1823
Bilusic M, Gulley JL (2012) Endpoints, patient selection, and biomarkers in the design of clinical trials for cancer vaccines. Cancer Immunol Immunother 61(1):109–117. doi:10.1007/s00262-011-1141-0
(2011) Guidance for industry: clinical considerations for therapeutic cancer vaccines. Department of Health and Human Services, Food and Drug Administration 76:68768–68769. https://federalregister.gov/a/2011-28726
Bland JM, Altman DG (2004) The logrank test. BMJ 328(7447):1073. doi:10.1136/bmj.328.7447.1073
Sposto R, Stablein D, Carter-Campbell S (1997) A partially grouped logrank test. Stat Med 16(6):695–704
Klein JP, Logan B, Harhoff M, Andersen PK (2007) Analyzing survival curves at a fixed point in time. Stat Med 26(24):4505–4519. doi:10.1002/sim.2864
Kalbfleisch J, Prentice R (2002) The statistical analysis of failure time data, 2nd edn. Wiley, New York
Logan BR, Klein JP, Zhang MJ (2008) Comparing treatments in the presence of crossing survival curves: an application to bone marrow transplantation. Biometrics 64(3):733–740. doi:10.1111/j.1541-0420.2007.00975.x
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thorén, F.B., Anderson, H. & Strannegård, Ö. Late divergence of survival curves in cancer immunotherapy trials: interpretation and implications. Cancer Immunol Immunother 62, 1547–1551 (2013). https://doi.org/10.1007/s00262-013-1458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1458-y