Abstract
Purpose
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immunosuppressive cells that are upregulated in cancer. Little is known about the prevalence and importance of MDSC in pancreas adenocarcinoma (PA).
Experimental design
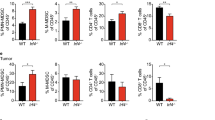
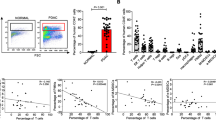
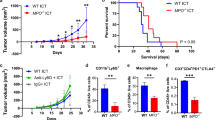
Peripheral blood, bone marrow, and tumor samples were collected from pancreatic cancer patients, analyzed for MDSC (CD15+CD11b+) by flow cytometry and compared to cancer-free controls. The suppressive capacity of MDSC (CD11b+Gr-1+) and the effectiveness of MDSC depletion were assessed in C57BL/6 mice inoculated with Pan02, a murine PA, and treated with placebo or zoledronic acid, a potent aminobisphosphonate previously shown to target MDSC. The tumor microenvironment was analyzed for MDSC (Gr1+CD11b+), effector T cells, and tumor cytokine levels.
Results
Patients with PA demonstrated increased frequency of MDSC in the bone marrow and peripheral circulation which correlated with disease stage. Normal pancreas tissue showed no MDSC infiltrate, while human tumors avidly recruited MDSC. Murine tumors similarly recruited MDSC that suppressed CD8+ T cells in vitro and accelerated tumor growth in vivo. Treatment with zoledronic acid impaired intratumoral MDSC accumulation resulting in delayed tumor growth rate, prolonged median survival, and increased recruitment of T cells to the tumor. This was associated with a more robust type 1 response with increased levels of IFN-γ and decreased levels of IL-10.
Conclusions
MDSC are important mediators of tumor-induced immunosuppression in pancreatic cancer. Inhibiting MDSC accumulation with zoledronic acid improves the host anti-tumor response in animal studies suggesting that efforts to block MDSC may represent a novel treatment strategy for pancreatic cancer.







Similar content being viewed by others
References
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. doi:10.1038/nri2506
Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V (2010) Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. doi:10.1016/j.immuni.2010.05.010
Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V (2008) Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 222:162–179. doi:10.1111/j.1600-065X.2008.00602.x
Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP (2009) Population alterations of L: -arginase- and inducible nitric oxide synthase-expressed CD11b(+)/CD14 (−)/CD15 (+)/CD33 (+) myeloid-derived suppressor cells and CD8 (+) T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. doi:10.1007/s00432-009-0634-0
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25(18):2546–2553. doi:10.1200/JCO.2006.08.5829
Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC (2005) Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med 202(7):931–939. doi:10.1084/jem.20050715
Schmielau J, Finn OJ (2001) Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 61(12):4756–4760
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 166(1):678–689
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI (2000) Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 6(5):1755–1766
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2008) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. doi:10.1007/s00262-008-0523-4
Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 70(1):68–77. doi:10.1158/0008-5472.CAN-09-2587
Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 69(4):1553–1560. doi:10.1158/0008-5472.CAN-08-1921
Li H, Han Y, Guo Q, Zhang M, Cao X (2009) Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 182(1):240–249
Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S (2009) Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol 183(2):937–944. doi:10.4049/jimmunol.0804253
Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 182(9):5693–5701. doi:10.4049/jimmunol.0900092
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111(8):4233–4244. doi:10.1182/blood-2007-07-099226
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 14(24):8270–8278. doi:10.1158/1078-0432.CCR-08-0165
Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J (2008) Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 181(1):346–353
Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH (2006) Gr-1+ CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66(2):1123–1131. doi:10.1158/0008-5472.CAN-05-1299
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC (2005) Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65(8):3044–3048. doi:10.1158/0008-5472.CAN-04-4505
Sinha P, Clements VK, Ostrand-Rosenberg S (2005) Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol 174(2):636–645
Sinha P, Clements VK, Ostrand-Rosenberg S (2005) Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res 65(24):11743–11751. doi:10.1158/0008-5472.CAN-05-0045
Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V (2004) Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother 53(2):64–72. doi:10.1007/s00262-003-0443-2
Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC (2004) Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 64(16):5839–5849. doi:10.1158/0008-5472.CAN-04-0465
Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM (2002) Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 168(2):689–695
Schmielau J, Nalesnik MA, Finn OJ (2001) Suppressed T-cell receptor zeta chain expression and cytokine production in pancreatic cancer patients. Clin Cancer Res 7(3 Suppl):933s–939s
Serafini P, Borrello I, Bronte V (2006) Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol 16(1):53–65. doi:10.1016/j.semcancer.2005.07.005
Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI (2010) Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol 184(6):3106–3116. doi:10.4049/jimmunol.0902661
Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL (2011) Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med 9:90. doi:10.1186/1479-5876-9-90
Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM, Kusmartsev S (2011) Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. doi:10.1002/ijc.26123
Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, Lang S (2011) Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol 89(2):311–317. doi:10.1189/jlb.0310162
Lechner MG, Liebertz DJ, Epstein AL (2010) Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 185(4):2273–2284. doi:10.4049/jimmunol.1000901
Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC (2011) Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets 11(6):734–751
Chioda M, Peranzoni E, Desantis G, Papalini F, Falisi E, Solito S, Mandruzzato S, Bronte V (2011) Myeloid cell diversification and complexity: an old concept with new turns in oncology. Cancer Metastasis Rev 30(1):27–43. doi:10.1007/s10555-011-9268-1
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300. doi:10.3322/caac.20073
Liyanage UK, Goedegebuure PS, Moore TT, Viehl CT, Moo-Young TA, Larson JW, Frey DM, Ehlers JP, Eberlein TJ, Linehan DC (2006) Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother 29(4):416–424. doi:10.1097/01.cji.0000205644.43735.4e
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169(5):2756–2761
Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, Eberlein TJ, Hsieh CS, Linehan DC (2009) Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 182(3):1746–1755
Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, Greten TF (2009) Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology 128(1):141–149. doi:10.1111/j.1365-2567.2009.03105.x
Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH (2007) Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 67(19):9518–9527. doi:10.1158/0008-5472.CAN-07-0175
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 15(6):2148–2157. doi:10.1158/1078-0432.CCR-08-1332
Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH (2008) Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 111(1):219–228. doi:10.1182/blood-2007-04-086835
Jia W, Jackson-Cook C, Graf MR (2010) Tumor-infiltrating, myeloid-derived suppressor cells inhibit T cell activity by nitric oxide production in an intracranial rat glioma + vaccination model. J Neuroimmunol 223(1–2):20–30. doi:10.1016/j.jneuroim.2010.03.011
Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Monkkonen H, Boccadoro M, Forni G, Musiani P, Bosia A, Cavallo F, Massaia M (2009) Zoledronic acid repolarizes tumor-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. doi:10.1111/j.1582-4934.2009.00926.x
Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP (2007) Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res 67(23):11438–11446. doi:10.1158/0008-5472.CAN-07-1882
Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rucklinger E, Greil R, Marth C (2009) Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 360(7):679–691. doi:10.1056/NEJMoa0806285
Diel IJ, Solomayer EF, Bastert G (2000) Bisphosphonates and the prevention of metastasis: first evidences from preclinical and clinical studies. Cancer 88(12 Suppl):3080–3088. doi:10.1002/1097-0142(20000615)88:12+<3080::AID-CNCR27>3.0.CO;2-W
Marten A, Lilienfeld-Toal M, Buchler MW, Schmidt J (2007) Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. J Immunother 30(4):370–377. doi:10.1097/CJI.0b013e31802bff16
Tassone P, Tagliaferri P, Viscomi C, Palmieri C, Caraglia M, D’Alessandro A, Galea E, Goel A, Abbruzzese A, Boland CR, Venuta S (2003) Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br J Cancer 88(12):1971–1978. doi:10.1038/sj.bjc.6600986
Ferretti G, Fabi A, Carlini P, Papaldo P, Cordiali Fei P, Di Cosimo S, Salesi N, Giannarelli D, Alimonti A, Di Cocco B, D’Agosto G, Bordignon V, Trento E, Cognetti F (2005) Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology 69(1):35–43. doi:10.1159/000087286
Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V, Tocchini M, Bonsignori M, Tonini G (2003) Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res 9(8):2893–2897
Santini D, Vincenzi B, Galluzzo S, Battistoni F, Rocci L, Venditti O, Schiavon G, Angeletti S, Uzzalli F, Caraglia M, Dicuonzo G, Tonini G (2007) Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res 13(15 Pt 1):4482–4486. doi:10.1158/1078-0432.CCR-07-0551
Tsagozis P, Eriksson F, Pisa P (2008) Zoledronic acid modulates antitumoral responses of prostate cancer-tumor associated macrophages. Cancer Immunol Immunother 57(10):1451–1459. doi:10.1007/s00262-008-0482-9
Wolf AM, Rumpold H, Tilg H, Gastl G, Gunsilius E, Wolf D (2006) The effect of zoledronic acid on the function and differentiation of myeloid cells. Haematologica 91(9):1165–1171
Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, Sporn MB, Liby KT, Rawal B, Lee JH, Gabrilovich DI (2010) Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res 16(6):1812–1823. doi:10.1158/1078-0432.CCR-09-3272
Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP Jr, Schabel FM Jr (1984) Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res 44(2):717–726
Linehan DC, Tan MC, Strasberg SM, Drebin JA, Hawkins WG, Picus J, Myerson RJ, Malyapa RS, Hull M, Trinkaus K, Tan BR Jr (2008) Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution phase II study. Ann Surg 248(2):145–151. doi:10.1097/SLA.0b013e318181e4e9
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966. doi:10.1200/JCO.2006.07.9525
Picozzi VJ, Kozarek RA, Traverso LW (2003) Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg 185(5):476–480
Linehan DC, Goedegebuure PS (2005) CD25+ CD4+ regulatory T-cells in cancer. Immunol Res 32(1–3):155–168. doi:10.1385/IR:32:1-3:155
Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V (2010) Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 40(1):22–35. doi:10.1002/eji.200939903
Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, Feng ZH (2007) CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett 252(1):86–92. doi:10.1016/j.canlet.2006.12.012
Zhang J, Patel L, Pienta KJ (2010) CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev 21(1):41–48. doi:10.1016/j.cytogfr.2009.11.009
Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC (2011) Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med 208(2):251–260. doi:10.1084/jem.20101700
Greenbaum AM, Link DC (2011) Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 25(2):211–217. doi:10.1038/leu.2010.248
Eash KJ, Greenbaum AM, Gopalan PK, Link DC (2010) CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest 120(7):2423–2431. doi:10.1172/JCI41649
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22(2):238–244. doi:10.1016/j.coi.2010.01.021
Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, Parney IF (2010) Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol 12(4):351–365. doi:10.1093/neuonc/nop023
Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL (2011) In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol 41(3):749–759. doi:10.1002/eji.201041069
Giraudo E, Inoue M, Hanahan D (2004) An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114(5):623–633. doi:10.1172/JCI22087
Aft R (2011) Bisphosphonates in breast cancer: antitumor effects. Clin Adv Hematol Oncol 9(4):292–299
Weiss HM, Pfaar U, Schweitzer A, Wiegand H, Skerjanec A, Schran H (2008) Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos 36(10):2043–2049. doi:10.1124/dmd.108.021071
Zhang B, Zhang Y, Bowerman NA, Schietinger A, Fu YX, Kranz DM, Rowley DA, Schreiber H (2008) Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res 68(5):1563–1571. doi:10.1158/0008-5472.CAN-07-5324
Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC (2006) Depletion of CD4+ CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol 13(9):1252–1258. doi:10.1245/s10434-006-9015-y
Burris HA III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181(8):5791–5802
Acknowledgment
This study was supported by a grant from the National Institutes of Health (T32CA009621).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porembka, M.R., Mitchem, J.B., Belt, B.A. et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother 61, 1373–1385 (2012). https://doi.org/10.1007/s00262-011-1178-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1178-0