Abstract
MVA-BN®-HER2 is a new candidate immunotherapy designed for the treatment of HER-2-positive breast cancer. Here, we demonstrate that a single treatment with MVA-BN®-HER2 exerts potent anti-tumor efficacy in a murine model of experimental pulmonary metastasis. This anti-tumor efficacy occurred despite a strong tumor-mediated immunosuppressive environment characterized by a high frequency of regulatory T cells (Treg) in the lungs of tumor-bearing mice. Immunogenicity studies showed that treatment with MVA-BN®-HER2 induced strongly Th1-dominated HER-2-specific antibody and T-cell responses. MVA-BN®-HER2-induced anti-tumor activity was characterized by an increased infiltration of lungs with highly activated, HER-2-specific, CD8+CD11c+ T cells accompanied by a decrease in the frequency of Treg cells in the lung, resulting in a significantly increased ratio of effector T cells to Treg cells. In contrast, administration of HER2 protein formulated in Complete Freund’s Adjuvant (CFA) induced a strongly Th2-biased immune response to HER-2. However, this did not lead to significant infiltration of the tumor-bearing lungs by CD8+ T cells or the decrease in the frequency of Treg cells nor did it result in anti-tumor efficacy. In vivo depletion of CD8+ cells confirmed that CD8 T cells were required for the anti-tumor activity of MVA-BN®-HER2. Furthermore, depletion of CD4+ or CD25+ cells demonstrated that tumor-induced Treg cells promoted tumor growth and that CD4 effector cells also contribute to MVA-BN®-HER2-mediated anti-tumor efficacy. Taken together, our data demonstrate that treatment with MVA-BN®-HER2 controls tumor growth through mechanisms including the induction of Th1-biased HER-2-specific immune responses and the control of tumor-mediated immunosuppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MVA-BN®-HER2 is currently being developed as a candidate for breast cancer immunotherapy and has shown biological activity in ongoing Phase I clinical trials (NCT0048277). MVA-BN®-HER2 is a Modified Vaccinia Ankara-based recombinant vaccine vector derived from MVA-BN® (IMVAMUNE®), a highly attenuated clonal virus currently in development as a smallpox vaccine [1]. MVA-BN®-HER2 encodes a modified form of human epidermal growth factor receptor 2 (HER-2), referred to as HER2. HER2 comprises of the extracellular domains of HER-2 and was further modified to encode two promiscuous T helper cell epitopes from tetanus toxin. This modification has been shown to improve its immunogenicity in a tolerant environment [2].
Successful cancer immunotherapy will most likely require the induction of comprehensive innate and Th1 adaptive immune responses that provide effector mechanisms able to directly kill tumor cells while combating the inflammatory/immunosuppressive responses induced by the growing tumor. In this regard, MVA-BN® has been shown to confer protection in animal models of smallpox through the induction of strong Th1 adaptive as well as innate immune responses [3–7]. These intrinsic immune properties are beneficial features to mediate adequate immune responses to MVA-BN®-encoded transgenes expressing tumor targets. This was demonstrated herein, by comparing the induction of HER-2-specific immune responses mediated by either MVA-BN®-HER2 or HER2 formulated in Freund’s adjuvant. When tested in a murine model of experimental pulmonary metastasis (CT26-HER-2), MVA-BN®-HER2-mediated immune responses translated into potent anti-tumor efficacy, while HER2 formulated in Freund’s adjuvant had only modest effects.
Multiple cell types can exert immunosuppressive functions within tumors, thereby hampering the development of successful immunotherapies [8–12]. Among these, regulatory T cells (Treg) appear to play a critical role in the progression of a number of cancers including breast cancer [13–17]. Even if tumor-specific effector cells are initially recruited into the tumor, the recruitment of Treg cells into the same site can result in an unproductive anti-tumor response, and this is believed to play a major part in the failure of many cancer immunotherapies presently in development [14, 18]. Furthermore, recent research in mouse and human showed that, regardless of the overall number of Treg cells in the tumor, the local balance of Treg to effector T cells (Teff) is critical to the success of immunotherapy and ultimately survival [19–22]. The murine CT26 colon carcinoma cell line has been shown to be a potent inducer of immunosuppressive mechanisms [23–25] making it a good model to evaluate the interplay between anti-tumor immunity and tumor-induced immunosuppression. When injected intravenously (i.v.), CT26 cells give rise to experimental pulmonary metastasis while mediating the infiltration of tumor-bearing lungs by a large number of IL-10-secreting Treg [25]. Data described here demonstrate that MVA-BN®-HER2 treatment induced Th1-dominated HER-2-specific cellular and humoral immunity, reduced the amount of Treg cells in the tumor and altered the intratumoral balance of effector and regulatory T cells resulting in potent anti-tumor efficacy against CT26-HER-2 tumors. Our findings suggest that MVA-BN®-HER2 generated an anti-tumor response in line with the current paradigm of an “immune signature” [26] that could lead to protective immunity in humans and warrants further testing of MVA-BN®-HER2 in clinical trials.
Materials and methods
Animals, tumor cell lines, MVA-BN®-vectors and HER2 proteins
Female BALB/c (H-2d) mice were obtained from Harlan Sprague–Dawley, Inc. CT26-HER-2 is a cell line derived from an undifferentiated murine colon adenocarcinoma that was transduced with human HER-2/neu [27]. CT26-HER-2 cells were grown in IMDM + 10% bovine calf serum (Cellgro, Mediatech, VA). MVA-BN® and MVA-BN®-HER2 were produced for BNIT by Bavarian Nordic (BN; Martinsried, Germany) [28]. The HER2 transgene encodes the extracellular domains of HER-2 and a portion of the trans-membrane domain but lacks the intracellular domains. Furthermore, it encodes the T helper cell epitopes p2 and p30 from tetanus toxin [2]. The HER2 protein used in combination with CFA is similar to the protein expressed by the MVA-BN®-transgene with the exception that the trans-membrane domain (9 AA) is absent and the protein has a C-terminal His tag.
Analysis of immune responses
Serum antibody titers and the frequency of IFN-γ-producing T cells were determined by standard ELISA and ELISPOT, respectively. Functionality of the T cells was tested by in vivo CTL assay and intracellular IFN-γ stain (for details, see Online Resource “ESM_1_Materials and Methods” MOESM1: Supplemental material 1.).
Immunotherapy
5E5 CT26-HER-2 cells were injected i.v. into female BALB/c mice. On day four, mice were treated intraperitoneally (i.p.) with 10 μg HER2 in CFA or 5E7 TCID50 of MVA-BN® or MVA-BN®-HER2 unless indicated otherwise in the Figure legend. Fourteen days after tumor implantation, mice were euthanized, bled by cardiac puncture, and lungs and spleens were collected. For assessment of tumor burden, lungs were washed in PBS, tapped dry on a tissue and weighed. The tumor burden was calculated by subtracting the average lung weight of naïve mice from the average lung weight of tumor-challenged TBS-treated mice [29]. % tumor reduction was calculated as: ((average lung weight of treated group − naïve group)/(average lung weight of TBS-treated group − naïve group)) × 100.
In vivo depletions
Mice were injected i.p. with 100 μg of anti-CD4 (GK1.5), anti-CD8 (2.43), anti-CD25 (PC61) or isotype control antibody starting 3 days before tumor implantation and then every 2–3 days throughout the study. Alternatively, 250 μg of antibody was given weekly. Depletion was confirmed on days 1 and 8 (on spleens and lungs from 2 mice per group) and at the end of study when FACS analysis was performed.
Statistical analysis
Statistical significance between treatment groups was evaluated by One-Way ANOVA followed by Bonferroni’s Multiple Comparisons post-test. Log-rank test was performed for survival curves.
Results
Treatment with MVA-BN®-HER2 induces potent therapeutic efficacy against established CT26-HER-2 tumors
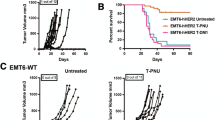
The immunotherapeutic efficacy of MVA-BN®-HER2 was evaluated in the CT26-HER-2 pulmonary metastasis model. Mice were challenged i.v. with 5E5 CT26-HER-2 cells and treated 3 days later with a single dose of either 1E7 TCID50 of the parental MVA-BN® vector, MVA-BN®-HER2 or 10 μg of HER2 protein formulated in Complete Freund’s Adjuvant (HER2+CFA). Control animals received TBS. Tumor burden of all mice was determined on day 15. As shown in Fig. 1a, lungs of TBS-treated mice were dramatically enlarged by the growing tumor and individual foci had already fused and were aggressively growing throughout the lung tissue (panels b and e). Strikingly, the lungs of animals treated with a single dose of MVA-BN®-HER2 had very small or no visible tumors by gross examination, and only a few small foci of tumor cells were visible by histology (panels c and f). While the average lung weight of tumor-challenged TBS-treated mice was almost threefold higher than that of naïve lungs, MVA-BN®-HER2 treatment had potent anti-tumor activity and inhibited tumor growth by 87% (P < 0.001, Fig. 1b). In contrast, tumor burden was not statistically different between mice of the TBS- and MVA-BN®-treated groups, demonstrating that anti-tumor efficacy was dependent on antigen-specific responses. Treatment with HER2+CFA showed non-statistically significant anti-tumor activity compared to no treatment (26% reduction in tumor burden). Hence, therapeutic intervention with MVA-BN®-HER2, but not with HER2+CFA (P < 0.001, Fig. 1b), resulted in the induction of protective HER-2-specific immune responses capable of suppressing aggressively growing HER-2-expressing tumors in mice. The anti-tumor activity of MVA-BN®-HER2 was further evaluated by monitoring mouse survival in two different model settings. In the first setting, mice were challenged with 5E5 CT26-HER-2 cells as described in Fig. 1b. Under these conditions, all control mice died by day 17 and the median survival of MVA-BN®-HER2-treated mice was approximately 25 days (see Fig. 3c). In the second and less aggressive setting, mice were challenged with lower tumor cell numbers (Fig. 1c). Under the latter conditions, 70% of mice treated with MVA-BN®-HER2 survived at least 120 days when treatment was started on day 4 (P < 0.0001). Furthermore, when the onset of treatment was delayed to day 8, significant protection was still observed with a survival rate of 30% 120 days after the tumor challenge (P < 0.0011).
Therapeutic anti-tumor efficacy of MVA-BN®, MVA-BN®-HER2 or HER2+CFA. a Representative pictures (a–c) and H&E-stained sections (40×, d–f) of lungs from naïve animals (no tumor challenge; top row) or tumor-challenged mice treated with TBS (middle row) or 1E7 TCID50 MVA-BN®-HER2 (bottom row). b Lung weights. Average lung weights (±SD) of each group and the % tumor reduction are indicated at the bottom of the graph (black bars demonstrate the median lung weight of each group). Mice were treated on day 4 with 1E7 TCID50 MVA-BN®-HER2, MVA-BN® or 10 μg HER2 protein formulated in CFA. Similar results were achieved in several repeat studies. c Survival study: Mice (n = 10/group) were challenged with 5E3 CT26-HER-2 cells on day 1 and treated q1 week × 4 with MVA-BN®-HER2 (1E7 TCID50) starting on day 4 (d4, 11, 18, 25) (D4) or on day 8 (d8, 15, 21, 28) (D8). TBS treatment was started on day 4
MVA-BN®-HER2 induces potent Th1-dominated immune responses resulting in infiltration of tumor-bearing lungs by highly activated, HER-2-specific, CD8+CD11c+ T cells
The modalities by which MVA-BN®-HER2 triggers protective immunity were evaluated in immunogenicity studies. As shown in Supplemental Figures 1 and 2, both MVA-BN®-HER2 and HER2+CFA treatments induced anti-HER-2 antibody and T-cell responses. However, these responses differed qualitatively in that MVA-BN®-HER2 induced a strongly Th1-dominated humoral response with an IgG2a/IgG1 ratio 1,000-fold higher than in HER2+CFA-immunized mice. Furthermore, treatment with MVA-BN®-HER2 induced five to tenfold higher frequencies of IFN-γ-producing, HER-2-specific T cells than HER2+CFA treatment. High amounts of TNF-α were also detected in supernatants of HER-2 restimulated splenocytes from MVA-BN®-HER2-treated animals, while HER2+CFA treatment induced the production of IL-5 (Supplemental Fig. 2e). Overall, these data are in line with the known Th1 adjuvant quality of the MVA-BN® vector.
To further identify the mechanism of MVA-BN®-HER2-mediated anti-tumor activity, lymphocytes infiltrating the lungs of tumor-challenged mice were characterized. The most striking result of treatment with MVA-BN®-HER2 or MVA-BN® was a strong accumulation of CD8 T cells. Indeed, 27.1 and 21.1% of lymphocytes were CD8+ in MVA-BN®-HER2- or MVA-BN®-treated animals, respectively. In contrast, CD8 T cells in lungs of TBS controls or lungs from HER2+CFA-treated animals were only marginally increased as compared to naïve lungs (Table 1).
While treatment with both viral vectors resulted in a high density of CD8 T cells in the lung, only MVA-BN®-HER2 markedly increased the number of activated effector/memory CD8 T cells as measured by high CD44 expression levels (40.4%, Table 1; Fig. 2a). In contrast, CD8 T cells from lungs treated with MVA-BN® showed similar levels of activation as naïve or HER2+CFA-treated mice. Notably, CD8 T-cell activation was reduced in tumor-bearing, TBS-treated animals, where only 7% of CD8 T cells were CD44high (a 53% reduction compared to naïve animals), possibly due to immunosuppressive activities provoked by the growing tumor.
Immunophenotyping of lung-infiltrating CD8+ T cells. FACS analysis of CD8+ T cells from lungs of mice treated as described in Fig. 1b. Similar results were achieved in 4 independent experiments using either 1E7 or 5E7 TCID50 of either virus. Gates a: CD3+ lymphocytes; b and c: CD3+CD8+ lymphocytes. d Functionality of p63-specific CD8 T cells isolated from lungs of MVA-BN®-HER2-treated animals. Panel a Lung weight in mg at day 11 after tumor challenge. Panel b Intracellular IFN-γ stain after 24 h in vitro restimulation with p63. Panel c p63-specific in vivo CTL activity. Lungs of 5 mice were pooled for both assays
In contrast to CD8 T cells, the frequency of CD4 T cells remained relatively similar in all groups, although it was somewhat increased in lungs of HER2+CFA-treated animals (Table 1). However, the frequency of effector/memory CD4 T cells was highest in lungs of mice treated with MVA-BN®-HER2 (42.5%) followed by treatment with HER2+CFA (35.7%). MVA-BN® only marginally increased CD4 T-cell activation above levels seen induced by the growing tumor (26.9 vs. 24.4% in TBS-treated animals).
Additional phenotyping of T cells infiltrating the lungs of MVA-BN®-HER2-treated animals revealed a high proportion of CD8+CD11c+ effector/memory cells. As shown in Fig. 2b, 18.8% of CD3+CD8+ T cells were CD11c+CD44high. This constitutes an approximately 20-fold increase in this specific cell population compared to naïve lungs or TBS-treated tumor-bearing animals. CD11c+CD44high cells were also detectable in the lungs of MVA-BN® or HER2+CFA-treated animals; however, the frequency of these cells was much lower (6.01 and 3.69%, respectively). Furthermore, the CD8+CD11c+-positive T cells found in the lung expressed NKG2D and KLRG-1, two markers often found on activated effector cells. As shown in panel 2 of Fig. 2b, the majority of CD8+CD11c+ T cells expressed NKG2D in each of the tumor-bearing groups, with a maximal percentage of 90% expressing NKG2D in the lungs of MVA-BN®-HER2-treated animals (Table 1). Interestingly, the expression of KLRG1 (a marker of CD8+ effector T cells commonly induced in viral infections [30]) was specific for CD8+CD11c+ T cells isolated from the lungs of MVA-BN® or MVA-BN®-HER2-treated animals.
To measure HER-2-specific T-cell responses, lymphocytes isolated from lungs or spleens were analyzed using a pentamer specific for the mouse MHC class I molecule Kd loaded with the immunodominant HER-2 peptide p63. As shown in Fig. 2c, the growing tumor alone did not induce significant amounts of p63-specific CD8 T cells (TBS 0.17 vs. 0.16% in naive lungs). In contrast, the percentage of p63-specific CD8+ T cells in the lungs of MVA-BN®-HER2-treated animals rose markedly to approximately 1%. Similar to the whole population of CD8+ T cells from the lungs of MVA-BN®-HER2-treated animals, 33% of p63-specific CD8+ T cells were CD11c+ and 43% were CD44high (data not shown). In comparison, only a slight elevation in p63-specific CD8+ T cells was observed in the lungs of MVA-BN® (0.31%) or HER2+CFA-treated mice (0.33%). Importantly, HER-2-specific CD8+ T cells appeared to accumulate specifically in tumor-bearing lungs since p63 staining was not detected above background in the spleens of any treatment group (data not shown). The functionality of the p63-specific CD8 T cells in the lungs of MVA-BN®-HER2-treated mice was tested during the effector phase of the response (6–7 days post-treatment). As shown in Fig. 2d (panel a), the tumor burden was similar between TBS- and MVA-BN®-HER2-treated groups at this time point (day 11). Restimulation with the p63 peptide resulted in the production of IFN-γ and CD107 by CD8+ T cells only in cells from the lungs of MVA-BN®-HER2-treated animals (Fig. 2d panel b). Furthermore, p63-labeled target cells were killed in vivo by tumor-challenged MVA-BN®-HER2-treated animals (Fig. 2d panel c). Notably, when non-challenged mice were treated with MVA-BN®-HER2, p63-specific T cells could only be found in the spleens (data not shown) but not in the lungs of this group of mice, demonstrating that the tumor is required for the homing of p63-specific T cells to the tumor. Taken together, these data demonstrate that MVA-BN®-HER2 treatment led to tumor infiltration by highly activated and functional (CD44high, NKG2D+, KLRG1+), HER-2-specific, CD8+CD11c+ T cells, while HER2+CFA treatment preferentially activated CD4+ T cells.
MVA-BN®-HER2 anti-tumor activity overrides the tumor-mediated accumulation of Treg cells in the lung
The reduced percentage of CD8+ T-cell activation in TBS-treated animals suggested that the growing tumor exerted immunosuppressive activities. In line with this, FoxP3 staining of lung-infiltrating CD4+ T cells revealed that the growth of CT26-HER-2 tumors in the lung was accompanied by a significant increase in the percentage of Treg cells from 2.22% (or 10.8% of CD4+ T cells) in naïve lungs to 5.79% (or 26.1% of CD4+ T cells) in tumor-bearing, TBS-treated mice (Table 2, line 1 and 4). Treatment of tumor-challenged mice with MVA-BN®-HER2 reduced the frequency of Treg cells to 3.44%. In contrast, treatment of tumor-bearing animals with MVA-BN® affected the frequency of Treg cells only marginally (4.89 vs. 5.79%), while HER2+CFA treatment slightly increased the frequency of Treg cells to 6.47% of lymphocytes.
To assess how treatment affected the balance between Teff cells and Treg cells in the lung, the CD4+ and CD8+ effector T cell to Treg cell ratios were calculated (Table 2). MVA-BN®-HER2 treatment significantly improved the ratio of both CD4+Teff/Treg cells and CD8+ Teff/Treg cells. Furthermore, the ratio of activated CD8+CD44high/Treg cells showed an even more dramatic 30-fold increase in the MVA-BN®-HER2-treated group as compared to the TBS-treated group and a fivefold increase as compared to naïve lungs. Treatment with the parental vector MVA-BN® more modestly increased the ratio of CD8+ Teff/Treg cells and CD8+CD44high/Treg cells as compared to TBS-treated mice but had little effect on CD4+Teff/Treg cell ratios. Treatment with HER2+CFA increased CD4+Teff/Treg ratios to a similar extent as MVA-BN® treatment but had only minor effects on CD8+ Teff/Treg ratios. These data demonstrate that treatment with MVA-BN®-HER2, but not with HER2+CFA, reduced the frequency of tumor-induced Treg cells in the lung and more importantly, led to a much higher ratio of effector to Treg cells, particularly with respect to CD8+ T cells.
In vivo depletion confirms that CD8+ cells are required for the anti-tumor activity of MVA-BN®-HER2 and identifies Treg cells as an immunosuppressive mechanism induced by the tumor
To further substantiate the role of CD8+ effector and Treg cells in this tumor model, mice were depleted of CD4+ or CD8+ cells beginning just prior to tumor challenge and throughout the entire course of the model. CD8 depletion reduced the anti-tumor activity of MVA-BN®-HER2 treatment from 99.6 to 42.4% (Fig. 3a). This difference was highly significant (P < 0.001) demonstrating the importance of CD8+ effector T cells in MVA-BN®-HER2-mediated anti-tumor efficacy. However, the anti-tumor activity of MVA-BN®-HER2 treatment was not completely abolished by CD8 depletion, as the mean lung weight from this group was still significantly smaller than those of CD8-depleted, TBS-treated mice (P < 0.05). This suggests that additional CD8-independent effector mechanisms contributed to the anti-tumor efficacy.
Effect of in vivo depletion of CD8+, CD4+ or CD25+ T cells on anti-tumor efficacy. a Tumor challenge and treatments were performed as described for Fig. 1. A single dose 5E7 TCID50 MVA-BN®-HER2 was given on day 4. CD4 or CD8 T cells were depleted by i.p. injection of anti-CD4, anti-CD8, anti-CD25 or isotype control antibody. Treatment with isotype control Ab had no effect on tumor growth (data not shown). CD4 and CD8 T-cell depletion was confirmed by FACS analysis (days 1, 8 and 15). Depletion was 100% complete throughout the study. Similar results were achieved in three independent studies. b Separate experiment evaluating the role of CD25+ T cells. Similar results were achieved in two independent studies. c Survival study: Mice (n = 15/group) were challenged with tumors on day 1 as described before and treated q1 week × 4 (days 4, 11, 18 and 25) with TBS or MVA-BN®-HER2 (1E7 TCID50) combined with CD4 or CD25 depletion. Antibodies were given on days −4, 3, 10, 17 and 24 at 250 μg/dose. Statistical analysis was performed by log-rank test
While CD8 depletion by itself had no effect on tumor growth (Fig. 3a), removal of CD4+ cells consistently resulted in a significant reduction in tumor burden even without MVA-BN®-HER2 treatment (Fig. 3a, b). Of note, combining CD4 depletion with MVA-BN®-HER2 treatment reliably decreased the anti-tumor activity of MVA-BN®-HER2 by 20–30% (Fig. 3a, b).
The decrease in tumor burden resulting from depletion of CD4+ cells in the absence of treatment implied that the increased percentage of CD4+CD25+Foxp3+ Treg cells present in the tumors (row 1, Table 2) play a key role in suppressing immune responses to the tumor. To test this directly, CD25+ cells were depleted by administration of an anti-CD25 mAb. CD25 depletion reduced tumor burden to a similar extent as CD4 depletion (35 and 48%, respectively, P > 0.05, Fig. 3b). This finding confirmed that the depletion of Treg cells led to the reduction in tumor burden in CD4-depleted mice and substantiates the significance of CD4+CD25+Foxp3+ Treg cells in this tumor model. However, the data also demonstrate that depletion of Treg cells alone does not result in effective tumor rejection.
The observed effects of CD4 or CD25 depletion on the therapeutic efficacy of MVA-BN®-HER2 were further distinguished by evaluating their effect on the survival rates of tumor-challenged mice. Mice were challenged with tumors and treated with MVA-BN®-HER2 weekly four times in the presence or absence of CD4 or CD25 depletion. All mice in the TBS group died between days 16 and 18. Treatment with MVA-BN®-HER2 significantly prolonged the median survival time from 17 to 24 days with one out of 15 mice surviving through day 60 when the study was terminated (Fig. 3c). CD4 depletion significantly reduced the median survival time from 24 to 21 days (P = 0.0081, Fig. 3c) supporting a role of CD4 effector cells in MVA-BN®-HER2-mediated anti-tumor immunity. In contrast, the depletion of CD25+ T cells prolonged the median survival time from 24 to 50 days resulting in significantly better survival than treatment with MVA-BN®-HER2 alone (P = 0.0011), presumably due to the more complete elimination of Treg cells.
Induction of HER-2-specific CD8 T cells requires treatment with MVA-BN®-HER2 even in the absence of Treg cells
Phenotypic analysis revealed a treatment-independent increase in overall CD8 T-cell activation in lungs of CD4+-depleted animals. Upon CD4 depletion, the percentage of activated CD8+ T cell rose from 5.6 to 26.7% in TBS-treated animals and from 12.8 to 29.6% in MVA-BN®-HER2-treated animals (Fig. 4a). The effect of CD4 depletion on the amplification of HER-2-specific CD8+ T cells, however, was dependent on treatment with MVA-BN®-HER2. No increase in HER-2-specific CD8+ T cells was observed in the context of CD4 depletion in TBS-treated mice (Fig. 4b). In contrast, the percentage of p63-specific CD8+ T cells reliably increased by two to threefold in lungs of CD4-depleted animals treated with MVA-BN®-HER2 (Fig. 4b).
Effect of CD4 or CD25 depletion on CD8 T-cell activation and frequency of HER-2-specific CD8 T cells. Animals were treated as described in legend to Fig. 3a, b. On day 15, single-cell suspensions were prepared from lungs and spleens for FACS analysis. a Percent of activated CD8+T cells in lung (black bars) and spleen (gray bars). b Percent Kd p63 pentamer+ cells of CD8+ T cells; similar results were achieved in three independent studies
In contrast to the depletion of all CD4+ cells, the functional depletion of Treg cells by treatment with anti-CD25 antibody [31] showed only minor effects on the activation of CD8+ T cells in TBS-treated animals (Fig. 4a). A modest increase in the frequency of activated CD8+ T cells from 12.8 to 19% in the lung and 14.4–31.2% in the spleen was observed in MVA-BN®-HER2-treated animals. Notably, the increase in p63-specific CD8+ T cells was more pronounced in MVA-BN®-HER2-treated animals combined with CD25 depletion than in combination with CD4 depletion (Fig. 4b), again suggesting a supportive role of CD4 T cells in MVA-BN®-HER2-mediated anti-tumor efficacy.
Discussion
Successful cancer immunotherapy has to accomplish two goals: first, the induction of an immune response which is capable of killing cancerous cells; second, to combat or reverse the inflammatory/immunosuppressive mechanisms exerted by the growing tumor that lead to subsequent down-regulation of therapy-induced responses. Pertaining to this challenge, the anti-tumor activity of MVA-BN®-HER2 was evaluated in a pre-clinical tumor model known to create a strong immunosuppressive environment able to restrain T-cell responses to both tumor-specific and bystander antigens [23–25]. Notably, a single treatment with MVA-BN®-HER2 showed potent anti-tumor activity against CT26-HER-2 lung metastasis despite the unfavorable immunosuppressive environment. The comparison of HER-2-specific humoral and cellular immune responses induced by treatment of mice with MVA-BN®-HER2 or HER2+CFA demonstrated that the MVA-BN® vector exerted potent Th-1 adjuvant activities. More importantly, the quality of the immune response is a crucial factor in cancer immunotherapy as only treatment with MVA-BN®-HER2 resulted in protective anti-tumor immunity in this aggressive tumor model, while treatment with HER2+CFA did not. Anti-tumor activity was accompanied by a high density of activated CD8+ T cells along with a clear reduction in the percentage of Treg cells in the lungs of animals treated with MVA-BN®-HER2, thus resulting in a crucial shift in the balance of Teff to Treg cells in favor of effector T cells [22]. Importantly, the tumor-infiltrating HER-2-specific CD8 Teff cells were functional as demonstrated by in vivo CTL activity and intracellular IFN-γ production. These cells were found in the spleen in MVA-BN®-HER2-treated non-challenged animals and homed to the lungs in tumor-challenged mice. Immunotherapy with MVA-BN®-HER2 also showed a significant anti-tumor efficacy in a solid CT26-HER2 tumor model (see Supplemental Fig. 3). In this model, epitope spreading to other tumor-associated antigens and long-lasting CTL memory was demonstrated.
A high percentage of CD8 T cells found in lungs of MVA-BN®-HER2- or MVA-BN®-treated mice expressed the markers CD11c, NKG2D and KLRG1. The expression of the myeloid marker CD11c on CD8 T cells has been described following acute infections with several viruses including vaccinia [32]. In general, all CD8+CD11c+ T cells also expressed elevated levels of CD44 and have been described in virally infected mice as activated, virus-specific, cytotoxic T cells that are much more effective at controlling viral infections than CD8+ T cells negative for CD11c [32–34]. Interestingly, CD8+CD11c+ T cells have also been identified as effectors of anti-4-1BB-mediated tumor suppression in several mouse tumor immunotherapy models using agonistic anti-4-1-BB antibodies [35–37]. Similar to the results described here, both NKG2D and KLRG-1 were highly expressed on anti-4-1-BB-induced CD8+CD11c+ T cells, and they preferentially accumulated in the tumor tissue. Together, these data suggest that CD8+CD11c+ T cells found in tumor-bearing lungs are a mixture of highly activated MVA virus- or tumor-specific CD8+ effector/memory T cells.
The lung in particular is an immunosuppressive environment in which the constitutive production of IL-10 is necessary to protect the integrity of the delicate and highly vascularized tissue. Growing CT26 tumors enhance this already immunosuppressive environment resulting in the recruitment of CD4+CD25+FoxP3+ Treg cells [25]. Natural CD4+CD25+ Treg play a critical role in the progression of a number of cancers [17] and are believed to play a major part in the failure of many immunotherapies against tumors in mice [24, 38–40] and men [18]. We found that the presence of CT26-HER-2 tumors in the lung consistently increased the percentage of Treg cells by approximately threefold. The fact that a single treatment with MVA-BN®-HER2 significantly reduced the frequency of Treg cells by up to 70% demonstrates that treatment with MVA-BN®-HER2 can override the potent effects of CD4+CD25+FoxP3+ Treg cells in establishing conditions conducive to tumor growth, even in the immunosuppressive environment of the lung. In stark contrast, treatment with HER2+CFA resulted in a further increase in the percentage of Treg cells in the lung and failed to induce any significant anti-tumor efficacy in this model. In vivo depletion experiments further confirmed that the importance of Treg cells in this tumor model as depletion of either CD4+ T cells or CD25+ T cells resulted in a similar partial reduction in tumor burden. MVA-BN®-HER2 therapy was necessary for the expansion of p63-specific CD8 T cells in both CD4- and CD25-depleted animals, demonstrating that the tumor alone was not able to induce HER-2-specific T cells even in the absence of Treg cells. However, phenotypic analysis of lung-infiltrating T cells from mice depleted of either CD25+ or CD4+ T cells revealed interesting differences. While CD4 depletion resulted in an increase in CD8 T-cell activation irrespective of treatment with MVA-BN®-HER2, the depletion of CD25+ cells only increased CD8 T-cell activation in MVA-BN®-HER2-treated mice. Although the increased activation of CD8 T cells could be responsible for the reduced tumor burden observed in CD4-depleted mice, it is unlikely that CD8+ T cells are responsible for the reduced tumor size observed in TBS-treated CD25-depleted mice. This could suggest that the absence of Treg cells might relieve immunosuppressive mechanisms that allow for the activation of cell types other than CD8 T cells. Two likely candidates are CD4 T cells or innate immune effector cells such as NK cells. We are currently investigating the role of CD4 T cells and innate immune mechanisms in MVA-BN®-HER2-mediated anti-tumor efficacy.
In cancer immunotherapy, a broad response involving all arms of the immune system is desired. Repeated treatment of mice with MVA-BN®-HER2 resulted in the induction of robust anti-HER-2 antibody responses (supplemental data Fig. 1). Using trastuzumab as a standard, we determined that these titers correlate with 50–500 μg HER-2-specific antibody/mL serum (data not shown), a level within the range of that found in patients treated with trastuzumab [41, 42]. Although HER-2-specific antibodies do not seem to play a protective role in this particular mouse tumor model, they can be important in other models (our own unpublished observations) and a role for antibody-mediated rejection of HER-2-positive tumors has been well established by the therapeutic activity of trastuzumab. Several other monoclonal antibodies have also demonstrated clinical effectiveness in a variety of malignancies through mechanisms such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) [43, 44]. Antibody response induced by MVA-BN®-HER2 showed a strong Th1 bias with IgG2a to IgG1 ratios 500- to 1,000-fold higher than in mice immunized with HER2+CFA. Since Th1 isotypes are thought to be the most efficient mediators of ADCC and CDC, the humoral responses produced by MVA-BN®-HER2 treatment are desirable and may provide therapeutic activity in human patients.
In conclusion, the data presented here demonstrate that a single treatment with MVA-BN®-HER2 was able to simultaneously induce Th1-dominated HER-2-specific immune responses and control tumor-induced immunosuppression resulting in potent anti-tumor efficacy. These preclinical results and the excellent safety profile and immunogenicity of MVA-BN® even in immune-compromised patients (4–6) provide further support for the development of MVA-BN®-HER2 for cancer immunotherapy. Notably, the immune responses induced by immunotherapy with MVA-BN®-HER2 are in line with a recently described distinct “immune signature” necessary for the inhibition of a variety of solid tumors treated with different immunotherapeutic strategies including breast cancer [26, 45–48]. Two hallmark features of this signature are the generation of a tumor-specific Th1 responses and a high density of T cells in the tumor parenchyma. The ability of MVA-BN®-HER2 to induce HER-2-specific immune responses in humans is currently being assessed in Phase I/II clinical trials in HER-2-positive breast cancer patients (NCT00485277).
References
Kennedy JS, Greenberg RN (2009) IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines 8:13–24
Renard V, Sonderbye L, Ebbehoj K, Rasmussen PB, Gregorius K et al (2003) HER-2 DNA and protein vaccines containing potent Th cell epitopes induce distinct protective and therapeutic antitumor responses in HER-2 transgenic mice. J Immunol 171:1588–1595
Brutkiewicz RR, Klaus SJ, Welsh RM (1992) Window of vulnerability of vaccinia virus-infected cells to natural killer (NK) cell-mediated cytolysis correlates with enhanced NK cell triggering and is concomitant with a decrease in H-2 class I antigen expression. Nat Immun 11:203–214
Dokun AO, Chu DT, Yang L, Bendelac AS, Yokoyama WM (2001) Analysis of in situ NK cell responses during viral infection. J Immunol 167:5286–5293
Dokun AO, Kim S, Smith HR, Kang HS, Chu DT et al (2001) Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2:951–956
Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC et al (2004) Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182–185
Wyatt LS, Earl PL, Eller LA, Moss B (2004) Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A 101:4590–4595
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
de Visser KE (2008) Spontaneous immune responses to sporadic tumors: tumor-promoting, tumor-protective or both? Cancer Immunol Immunother 57:1531–1539
de Visser KE, Coussens LM (2006) The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol 13:118–137
Gross S, Geldmacher A, Sharav T, Losch F, Walden P (2009) Immunosuppressive mechanisms in cancer: consequences for the development of therapeutic vaccines. Vaccine 27:3398–3400
Egeblad M, Nakasone ES, Werb Z (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18:884–901
Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG et al (2002) Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 168:4272–4276
Barnett BG, Ruter J, Kryczek I, Brumlik MJ, Cheng PJ et al (2008) Regulatory T cells: a new frontier in cancer immunotherapy. Adv Exp Med Biol 622:255–260
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G et al (2007) CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 13:6947–6958
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H et al (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61:4766–4772
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E et al (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Quezada SA, Peggs KS, Simpson TR, Allison JP (2011) Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev 241:104–118
Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP (2009) Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 206:1717–1725
Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR et al (2008) Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med 205:2125–2138
Quezada SA, Peggs KS, Curran MA, Allison JP (2006) CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 116:1935–1945
Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A et al (2003) CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol 171:5931–5939
Grimm M, Gasser M, Bueter M, Strehl J, Wang J et al (2010) Evaluation of immunological escape mechanisms in a mouse model of colorectal liver metastases. BMC Cancer 10:82
Jarnicki AG, Lysaght J, Todryk S, Mills KH (2006) Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol 177:896–904
Disis ML, Park KH (2009) Immunomodulation of breast cancer via tumor antigen specific Th1. Cancer Res Treat 41:117–121
Penichet ML, Challita PM, Shin SU, Sampogna SL, Rosenblatt JD et al (1999) In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Lab Anim Sci 49:179–188
Li Z, Ling L, Liu X, Laus R, Delcayre A (2010) A flow cytometry-based immuno-titration assay for rapid and accurate titer determination of modified vaccinia Ankara virus vectors. J Virol Methods 169:87–94
Jenkins DE, Oei Y, Hornig YS, Yu SF, Dusich J et al (2003) Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis 20:733–744
Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S et al (2008) Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 205:625–640
Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M et al (2006) Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol 176:3301–3305
Lin Y, Roberts TJ, Sriram V, Cho S, Brutkiewicz RR (2003) Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur J Immunol 33:2736–2743
Beyer M, Wang H, Peters N, Doths S, Koerner-Rettberg C et al (2005) The beta2 integrin CD11c distinguishes a subset of cytotoxic pulmonary T cells with potent antiviral effects in vitro and in vivo. Respir Res 6:70
Kim SK, Schluns KS, Lefrancois L (1999) Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol 163:4125–4132
Kim YH, Choi BK, Oh HS, Kang WJ, Mittler RS et al (2009) Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy. Mol Cancer Ther 8:469–478
Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS (2008) Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res 68:7264–7269
Choi BK, Kim YH, Kang WJ, Lee SK, Kim KH et al (2007) Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy. Cancer Res 67:8891–8899
Jones E, Golgher D, Simon AK, Dahm-Vicker M, Screaton G et al (2004) The influence of CD25+ cells on the generation of immunity to tumour cell lines in mice. Novartis Found Symp 256:149–152; discussion 152–157, 259–169
Golgher D, Jones E, Powrie F, Elliott T, Gallimore A (2002) Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol 32:3267–3275
Jones E, Dahm-Vicker M, Golgher D, Gallimore A (2003) CD25+ regulatory T cells and tumor immunity. Immunol Lett 85:141–143
Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR (2003) Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol 21:283–290
Stemmler HJ, Schmitt M, Harbeck N, Willems A, Bernhard H et al (2006) Application of intrathecal trastuzumab (Herceptintrade mark) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep 15:1373–1377
Natsume A, Niwa R, Satoh M (2009) Improving effector functions of antibodies for cancer treatment: enhancing ADCC and CDC. Drug Des Devel Ther 3:7–16
Clark MR (1997) IgG effector mechanisms. Chem Immunol 65:88–110
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H et al (2007) Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res 9:R65
Roepman P, Jassem J, Smit EF, Muley T, Niklinski J et al (2009) An immune response enriched 72-gene prognostic profile for early-stage non-small-cell lung cancer. Clin Cancer Res 15:284–290
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Acknowledgments
The authors thank Tobias Njoku and Fareed Yahya for their expert animal handling and assistance in husbandry.
Conflict of interest
The authors declare that they are employees and stock holders of BN ImmunoTherapeutics.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Stefanie J. Mandl and Ryan B. Rountree contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mandl, S.J., Rountree, R.B., Dalpozzo, K. et al. Immunotherapy with MVA-BN®-HER2 induces HER-2-specific Th1 immunity and alters the intratumoral balance of effector and regulatory T cells. Cancer Immunol Immunother 61, 19–29 (2012). https://doi.org/10.1007/s00262-011-1077-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1077-4