Abstract
Background
Anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibodies, such as ipilimumab, have generated measurable immune responses to Melan-A, NY-ESO-1, and gp100 antigens in metastatic melanoma. Vaccination against such targets has potential for immunogenicity and may produce an effector-memory T-cell response.
Methods
To determine the effect of CTLA-4 blockade on antigen-specific responses following vaccination, in-depth immune monitoring was performed on three ipilimumab-treated patients prevaccinated with gp100 DNA (IMF-24), gp100209–217 and tyrosinase peptides plus GM-CSF DNA (IMF-32), or NY-ESO-1 protein plus imiquimod (IMF-11); peripheral blood mononuclear cells were analyzed by tetramer and/or intracellular cytokine staining following 10-day culture with HLA-A*0201-restricted gp100209–217 (ITDQVPFSV), tyrosinase369–377 (YMDGTMSQV), or 20-mer NY-ESO-1 overlapping peptides, respectively. Tumors from IMF-32 were analyzed by immunohistochemistry to help elucidate mechanism(s) underlying tumor escape.
Results
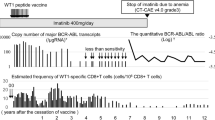
Following vaccination, patients generated weak to no CD4+ or CD8+ T-cell response specific to the vaccine antigen but demonstrated increases in effector-memory (CCR7loCD45RAlo) tetramer+CD8+ T cells. After ipilimumab induction, patients experienced a robust, although sometimes transient, antigen-specific response for gp100 (IMF-32 and IMF-24) or NY-ESO-1 (IMF-11) and produced polyfunctional intracellular cytokines. Primary and metastatic tumors expressed tyrosinase but not gp100 or class I/II MHC molecules.
Conclusion
Vaccination induced a measurable antigen-specific T-cell response that increased following CTLA-4 blockade, potentially “boosting” the vaccine-primed response. Tumor escape may be related to antigen loss or lack of MHC expression necessary for immune activity. These results in a limited number of patients support the need for further research into combining vaccination with ipilimumab and provide insight into mechanisms underlying tumor escape.



Similar content being viewed by others
Abbreviations
- ALC:
-
Absolute lymphocyte count
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- H&E:
-
Hematoxylin and eosin
- ICOS:
-
Inducible co-stimulator
- ICS:
-
Intracellular cytokine staining
- IHC:
-
Immunohistochemistry
- PBMC:
-
Peripheral blood mononuclear cell
- WBC:
-
White blood cell
References
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Atkins MB, Lotze MT, Dutcher JP et al (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17:2105–2116
Eigentler TK, Caroli UM, Radny P, Garbe C (2003) Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol 4:748–759
Chapman PB, Einhorn LH, Meyers ML et al (1999) Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 17:2745–2751
Petrella T, Quirt I, Verma S et al (2007) Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev 33:484–496
Karbach J, Gnjatic S, Bender A et al (2010) Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51: association with survival. Int J Cancer 126:909–918
Wolchok JD, Yuan J, Houghton AN et al (2007) Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther 15:2044–2050
Perales MA, Yuan J, Powel S et al (2008) Phase I/II study of GM-CSF DNA as an adjuvant for a multipeptide cancer vaccine in patients with advanced melanoma. Mol Ther 16:2022–2029
Spaner DE, Astsaturov I, Vogel T et al (2006) Enhanced viral and tumor immunity with intranodal injection of canary pox viruses expressing the melanoma antigen, gp100. Cancer 106:890–899
Smith CL, Dunbar PR, Mirza F et al (2005) Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int J Cancer 113:259–266
Lacelle MG, Jensen SM, Fox BA (2009) Partial CD4 depletion reduces regulatory T-cells induced by multiple vaccinations and restores therapeutic efficacy. Clin Cancer Res 15:6881–6890
Nicholaou T, Ebert LM, Davis ID et al (2009) Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res 15:2166–2173
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T-cells to stimulation. J Exp Med 182:459–465
Salomon B, Lenschow DJ, Rhee L et al (2000) B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T-cells that control autoimmune diabetes. Immunity 12:431–440
Karandikar NJ, Vanderlugt CL, Walunas TL et al (1996) CTLA-4: a negative regulator of autoimmune disease. J Exp Med 184:783–788
Brunner MC, Chambers CA, Chan FK et al (1999) CTLA-4-mediated inhibition of early events of T-cell proliferation. J Immunol 162:5813–5820
van Elsas A, Hurwitz AA, Allison JP (1999) Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190:355–366
Maker AV, Attia P, Rosenberg SA (2005) Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol 175:7746–7754
Quezada SA, Peggs KS, Curran MA, Allison JP (2006) CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T-cells. J Clin Invest 116:1935–1945
Quezada SA, Peggs KS, Simpson TR et al (2008) Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T-cell depletion against established melanoma. J Exp Med 205:2125–2138
Hodi FS, Mihm MC, Soiffer RJ et al (2003) Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 100:4712–4717
Phan GQ, Yang JC, Sherry RM et al (2003) Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 100:8372–8377
Lynch T, Neal J, Bondarenko I et al (2010) Phase 2 trial of ipilimumab (IPI) and paclitaxel/carboplatin (P/C) in first-line stage IIIb/IV non-small cell lung cancer (NSCLC). Presented at the 2010 European Society for Clinical Oncology (ESMO) Annual Meeting; October 8–12, 2010, Milan, Italy. Abstract 375PD
Slovin SF, Beer TM, Higano CS et al (2009) Initial phase II experience of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration resistant prostate cancer (mCRPC). Presented at the 2009 American Society for Clinical Oncology (ASCO) Meeting; May 29–June 2, 2009, Orlando, FL. Abstract 5138
Yuan J, Gnjatic S, Li H et al (2008) CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T-cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA 105:20410–20415
Klein O, Ebert LM, Nicholaou T et al (2009) Melan-A-specific cytotoxic T-cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res 15:2507–2513
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Fong L, Kwek SS, O’Brien S et al (2009) Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res 69:609–615
Ribas A, Comin-Anduix B, Chmielowski B et al (2009) Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res 15:6267–6276
Ku GY, Yuan J, Page DB et al (2010) Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 116:1767–1775
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Yuan J, Ku GY, Gallardo HF et al (2009) Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun 9:5
Barrow C, Browning J, MacGregor D et al (2006) Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 12:764–771
Carthon BC, Wolchok JD, Yuan J et al (2010) Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 16:2861–2871
Romero P, Zippelius A, Kurth I et al (2007) Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol 178:4112–4119
Casazza JP, Betts MR, Price DA et al (2006) Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med 203:2865–2877
Genescà M, Rourke T, Li J et al (2007) Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T-cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J Immunol 179:4732–4740
Duvall MG, Precopio ML, Ambrozak DA et al (2008) Polyfunctional T-cell responses are a hallmark of HIV-2 infection. Eur J Immunol 38:350–363
Precopio ML, Betts MR, Parrino J et al (2007) Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T-cell responses. J Exp Med 204:1405–1416
Adams S, O’Neill DW, Nonaka D et al (2008) Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol 181:776–784
Lin Y, Gallardo HF, Ku GY et al (2009) Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses. Cytotherapy 11:1–11
Vence L, Palucka AK, Fay JW et al (2007) Circulating tumor antigen-specific regulatory T-cells in patients with metastatic melanoma. Proc Natl Acad Sci USA 104:20884–20889
Thomson TM, Real FX, Murakami S et al (1988) Differentiation antigens of melanocytes and melanoma: analysis of melanosome and cell surface markers of human pigmented cells with monoclonal antibodies. J Invest Dermatol 90:459–466
US National Institutes of Health. www.ClinicalTrials.gov. http://www.clinicaltrials.gov/ct2/show/NCT00836407?term=ipilimumab&rank=6; http://www.clinicaltrials.gov/ct2/show/NCT00357461?term=ipilimumab&rank=26; http://www.clinicaltrials.gov/ct2/show/NCT00124670?term=ipilimumab&rank=42
Acknowledgments
We thank Dr. Immanuel Luescher from Tetramer Core, Lausanne Branch, Ludwig Institute of Cancer Research, for providing the tetramers; Cora Mariano from the MSKCC Departments of Pathology for the collection of tumor tissues; and Hematology for the lymphocyte counts. This work was supported by Swim Across America, the Experimental Therapeutics Center of MSKCC and Ludwig Foundation. JDW was supported by a Damon Runyon-Lilly Clinical Investigator Award. JY was supported by MSKCC Pilot Grant of Grant number P50AT002779 from the National Center for complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS). Editorial and writing assistance was provided by StemScientific with funding from Bristol-Myers Squibb Company.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is a Focussed Research Review based on a presentation given at the Tenth International Conference on Progress in Vaccination against Cancer (PIVAC 10), held in St. Catharine’s College, Cambridge, UK, September 27th–30th, 2010. It is part of a CII series of Focussed Research Reviews and meeting report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, J., Ginsberg, B., Page, D. et al. CTLA-4 blockade increases antigen-specific CD8+ T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother 60, 1137–1146 (2011). https://doi.org/10.1007/s00262-011-1011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1011-9