Abstract
We have developed a new vaccination strategy by using the Salmonella type III secretion system (T3SS) to translocate heterologous antigens into the cytosol of host cells. This leads to an efficient antigen-specific CD8 T cell induction. Recently, we have demonstrated the use of Salmonella’s T3SS for the immunoprophylaxis of a solid tumor. The murine fibrosarcoma WEHI 164 was transfected with the DNA sequence encoding the MHC class I-peptide p60217–225 from Listeria monocytogenes. In the present study, we used this tumor model to investigate the potential of vaccination with recombinant Salmonella in a therapeutic setting. BALB/c mice were subcutaneously challenged with WEHI-p60 cells. Simultaneously or 4 days later, these mice received either an orogastric or intravenous immunization with Salmonella translocating p60. Interestingly, 71–80% of the intravenously and 50–52% of the orogastrically immunized mice showed a complete tumor regression after 14 days. In addition, the distribution of tetramer-positive p60217–225-specific CD8 T cell subpopulations in blood and tumor tissue was analyzed. Co-staining with CD62L and CD127 revealed that the frequencies of p60217–225-specific effector and effector memory CD8 T cells in blood and in fibrosarcoma tissue were related to the kinetics of tumor regression. In summary, our study demonstrates that therapeutic vaccination with Salmonella leads to efficient induction of tumor-invading effector CD8 T cells that may result in significant tumor regression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical cancer immunotherapy has achieved encouraging, yet limited success. Tumors have evolved multiple mechanisms to evade the immune response, including antigen loss, downregulation of the major histocompatibility complex (MHC) and the production of immunosuppressive factors [10, 37, 39]. In addition, many tumors lack expression of co-stimulatory molecules critical for the activation of naïve T cells. Finally, tolerance mechanisms are operative in vivo to prevent T cell activation in response to tumor antigens that are expressed in many cases also in normal tissue. A strategy to circumvent part of these problems and to study particular aspects of cancer immunobiology includes the development of experimental tumor systems using tumors transfected with immunogenic model antigens of bacterial or viral origin [20, 22, 37]. Data compiled from both in vitro systems and animal models using either naturally occurring tumors or transfected cancer cells clearly show that CD8 and CD4 T cells play a pivotal role in the effective eradication of tumors [11, 14, 19]. Thus, the majority of cancer immunotherapy efforts are devoted to the stimulation of T cellular immune responses.
Attenuated recombinant Salmonella enterica serovar Typhimurium has emerged as a promising delivery system for foreign vaccine antigens [21]. Upon close contact with the eukaryotic cell, a type III secretion system (T3SS) encoded by the “Salmonella pathogenicity island 1” mediates Salmonella invasion of the host cell, where the bacterium resides within Salmonella-containing vacuoles. The T3SS is designed to translocate Salmonella type III effector proteins directly into the cytosol of target cells [8]. Our laboratory has focused its research on the genetic manipulation of attenuated Salmonella strains to endow them with the ability for efficient induction of MHC class I-restricted immune responses [28, 32]. We have developed a new vaccination strategy by using the Salmonella-T3SS to translocate microbial antigens directly into the cytosol of antigen-presenting cells. The immunodominant p60 antigen from Listeria monocytogenes was fused to the defined N-terminal translocation domain of the type III effector molecule Yersinia outer protein E (YopE) [33]. Translocation and cytosolic delivery of the chimeric YopE/p60 protein into macrophages led to efficient MHC class I-restricted antigen presentation of the p60 nonamer peptide p60217–225. As determined by enzyme-linked immunospot assay, mice orally vaccinated with a single dose of attenuated Salmonella expressing translocated YopE/p60 protein revealed high numbers of interferon-gamma (IFN-γ)-producing CD8 T cells reactive with p60217–225. In a more recent study, we demonstrated that the use of Salmonella’s T3SS to induce antigen-specific cytotoxic T cells is also an attractive strategy to develop vaccines for the immunoprophylaxis of tumors [27].For these experiments, we established an experimental tumor model in BALB/c mice [27]. The murine fibrosarcoma cell line WEHI 164 [13] was stably transfected with DNA encoding the immunodominant listerial MHC class I-restricted nonamer epitope p60217–225 [27]. In naïve mice, subcutaneous inoculation of these WEHI-p60 tumor cells resulted in progressive growth of a solid fibrosarcoma for approximately 14 days without inducing a measurable frequency of p60217–225-tetramer-positive CD8 T cells. However, in vitro antigen presentation assays revealed that WEHI-p60 cells are able to present p60217–225 via MHC class I molecules, although with a relatively weak efficiency. Thus, despite being transfected with an immunodominant bacterial antigen, this fibrosarcoma model resembles naturally occurring tumors that are often characterized by their weak immunogenicity. In further experiments, BALB/c mice received a single orogastric immunization with Salmonella that translocates YopE/p60 via its T3SS. Four weeks later, mice were challenged subcutaneously with WEHI-p60 tumor cells. In vivo protection studies revealed that 80% of these mice remained tumor free, whereas all animals of the non-vaccinated control group developed tumor growth. Taken together, our approach clearly demonstrated protection against a tumor in a prophylactic setting [27].
In this study, the potential of our vaccination strategy was evaluated with regard to a therapeutic intervention against cancer. Therefore, we used the above-described model and applied the YopE/p60 expressing Salmonella vaccine strain either simultaneously or 4 days after subcutaneous tumor injection.
Materials and methods
Plasmids, bacterial strains and growth conditions
The construction of plasmid pHR241 has been outlined in detail [33]. This low-copy number expression vector bears the genetic information for the translocated chimeric YopE1–138/p60130–477/M45 fusion protein under expression control of the lac promoter. M45 is derived from the E4–6/7 protein of adenovirus and its use for chimeric protein tagging has been described [33]. The above described plasmid was transformed into Salmonella enterica serovar Typhimurium strain SB824 by electroporation. Strain SB824 was engineered by introducing the sptP::kan mutant allele from strain SB237 into the ΔaroA strain SL3261 by P22HTint transduction [1, 32, 33]. Serovar Typhimurium was grown in Luria-Bertani medium supplemented with 0.3 M NaCl, pH 7.4, to allow expression of components and targets of the T3SS encoded by the Salmonella pathogenicity island 1, which mediates Salmonella invasion of host cells [33].
Stable transfection of fibrosarcoma cell line
Generation of WEHI-p60 cells has been outlined in detail [27]. To obtain WEHI-p60 cells, the H-2d fibrosarcoma WEHI 164 (ATCC # CRL-1751), a methylcolanthrene-induced tumor of BALB/c origin, was transfected with a double-stranded synthetic oligonucleotide (oligonucleotide A, 5′-CCGGTGCCACCATGAAATACGG TGTTTCTGTTCAAGACATTTGAG-3′; oligonucleotide B, 5′-GATCCTCAAATGTCTT GAACAGAAACACCGTATTTCATGGTGGCA-3′) encoding the p60217–225-epitope, which was inserted into the mammalian expression vector pIRESneo3 (BD Biosciences, Heidelberg, Germany). Cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine, 2-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml) and G418 (400 μg/ml) (Sigma, Deisenhofen, Germany). Slowly progressive in vivo growth was obtained by careful in vitro cell incubation to a density of maximum 70% and strict avoidance of medium acidification by continuous pH indicator controls [12]. No differences between WEHI 164 and WEHI 164-p60 growth could be observed in vivo or in vitro.
Generation and purification of H2-Kd tetramers
Tetrameric H2-Kd/p60217–225 complexes were generated as previously described [2]. Briefly, recombinant H2-Kd heavy chain and β2-microglobulin were expressed as insoluble inclusion bodies in Escherichia coli and were further purified. The H2-Kd heavy chain molecule was mutated to remove the transmembrane and cytosolic domain and to add a specific biotinylation site at the C-terminus. Purified proteins were refolded in vitro in the presence of high concentrations of synthetic peptides (Biosythan, Berlin, Germany) to form stable and soluble MHC/peptide complexes. Complexes were specifically biotinylated in vitro by adding the enzyme BirA, d-biotin and ATP. After further purification, biotinylated MHC/peptide complexes were multimerized with streptavidin-PE (SA-PE; Molecular Probes, Eugene, USA). Tetrameric complexes were purified by gel filtration and stored at 2–5 mg/ml at 4°C in phosphate-buffered saline (pH 8.0) containing 0.02% sodium azide, 1 μg/ml pepstatin, 1 μg/ml leupeptin and 0.5 ml EDTA.
Mice, in vivo tumor studies and immunization with recombinant Salmonella
Specific-pathogen-free female BALB/c mice, 6–8 weeks old, were purchased from Harlan–Winkelmann (Horst, Netherlands). For the experiments, animals were housed in groups of five mice under standard barrier conditions in individually ventilated cages (Tecniplast, Buguggiate, Italy). BALB/c mice received a subcutaneous injection of 5 × 106 WEHI-p60 cells in the flank on day 0. Mice given the fibrosarcoma cells were inspected on days 4, 5, 7, 10 and 14 for tumor growth and size. Tumor growth was measured by using a digital calliper to record maximum length and width. Values shown for tumor size (mm2) are the product of these two parameters per animal averaged over the total number of mice in the respective group. Animal experiments were approved by the German authorities and performed according to the legal requirements. No apparent suffering of mice occurred after tumor inoculation and all animals behaved normally without any signs of pain or other disorders.
To evaluate the therapeutic potential of our Salmonella-based vaccination, four different immunization strategies were performed. The recombinant Salmonella vaccine strain SB824 (pHR241) expressing translocated YopE/p60 was applied intravenously (1 × 106 colony-forming units, CFU) on day 0 (immunization group A, simultaneous application with WEHI-p60 cells) or on day 4 after tumor injection (immunization group B). Mice of immunization group C received SB824 (pHR241) orogastrically (5 × 108 CFU) by using round-bottom gavage needles on day 0, whereas animals of immunization group D were vaccinated orogastrically on day 4 after subcutaneous tumor application. Control mice were immunized with plasmidless SB824 (immunization group S) or received phosphate-buffered saline (non-immunized, NI), respectively. Each experiment was performed at least twice with similar results. Complete tumor regression was defined by the calliper detection threshold of 3 mm × 3 mm. Observation of these mice for a period of three more months did not reveal any signs of local or systemic tumor relapse. As previously published [27], this immunization approach leads to the generation of sufficient amounts of p60-specific memory T cells, providing successful protection against tumor rechallenge 4 weeks post-vaccination.
Preparation of cells from blood and tumor tissue
Blood samples were collected on day 14 after tumor injection. Cells from blood were harvested by dissociation through a wire mesh and lysis of erythrocytes with ammonium chloride and were subsequently resuspended in RP10+, which consists of RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine, HEPES (pH 7.5), 2-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml) and gentamicin (50 μg/ml).
On days 4, 5, 7, 10 and 14, fibrosarcoma tissue was removed from mice and single-cell suspensions were prepared by enzymatic digestion. Resected tumors were weighed, minced into small (1–2 mm3) pieces with a scalpel and immersed in 10 ml of digestion mixture (5% FBS in RPMI 1640, 0.5 mg/ml collagenase A, 0.2 mg/ml hyaluronidase, type V and 0.02 mg/ml DNase I) per 0.25 g of tumor tissue. This mixture was incubated at 37°C for 45 min on a rotating platform. The resulting cell suspension was filtered sequentially through 70- and 40-μm cell strainers and washed with 5% FBS in RPMI 1640. Red blood cells were lysed by brief incubation in 0.15 M ammonium chloride solution.
MHC tetramer staining and FACS analysis
The p60217–225-specific T cell populations from blood and tumor tissue were detected with phycoerythrin (PE)-conjugated, tetrameric MHC I/peptide complexes and concurrently stained for other surface molecules using directly conjugated monoclonal antibodies as described previously [2, 3]. Briefly, cells were incubated with ethidium bromide monoazide for live/dead discrimination in FACS-staining buffer (phosphate-buffered saline, pH 7.45, 0.5% bovine serum albumin and 0.02% sodium azide). Subsequently, cells were stained with the above-mentioned MHC class I tetramer and surface markers for 1 h. The following monoclonal antibodies were used: anti-CD8α (clone 53-5.8, PharMingen, Heidelberg, Germany), anti-CD62L (clone Mel-14, PharMingen) and anti-CD127 (clone A7R34, eBioscience, San Diego, CA, USA). Cells were resuspended in staining buffer and fixed in 1% paraformaldehyde/phosphate-buffered saline (pH 7.45). Data were acquired on a FACSCanto (BD Biosciences, San Jose, CA, USA) and further analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Statistical analysis
The results are depicted as mean ± SD of triplicate determinations. Statistical significance of the results was checked with the non-parametric Mann–Whitney U test at the *p < 0.01, **p < 0.005 and ***p < 0.001 significance level. Correlation analysis was performed with the non-parametric Kendall tau rank correlation test. All tests were performed using the SPSS (SPSS, Chicago, IL, USA) and Microsoft Office Excel 2007 software.
Results
Efficient reduction of fibrosarcoma growth after intravenous or orogastric vaccination with recombinant Salmonella
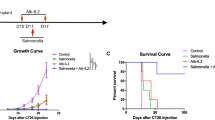
In this study, we wanted to evaluate the therapeutic potential of our recently developed Salmonella-based vaccination strategy [27]. We decided to apply four different immunization strategies (A–D) to BALB/c mice (Fig. 1a–d). Animals of all immunization groups received the fibrosarcoma WEHI-p60 subcutaneously on day 0. Tumor growth was measured between days 4 and 14. Mice of the immunization group A obtained an intravenous vaccination with SB824 (pHR241) on day 0 simultaneously with tumor injection (Fig. 1a, black triangle). Control mice of the immunization group S were immunized with plasmidless SB824 (Fig. 1a, black diamond) or remained non-immunized (NI) (Fig. 1a, black square). In Fig. 1a, it is demonstrated that in mice vaccinated with SB824 (pHR241) peak fibrosarcoma growth on day 7 (35 ± 6 mm2) was significantly reduced on day 10 (18 ± 6 mm2) and on day 14 (9 ± 5 mm2) after tumor injection. Interestingly, mice of the immunization group S revealed a transient tumor growth delay during the first days after vaccination. However, immunization with SB824 did not result in the reduction of WEHI-p60 growth on day 14 (63 ± 7 mm2) as compared to non-immunized mice (63 ± 9 mm2).
Regression of fibrosarcoma growth after therapeutic vaccination with recombinant Salmonella. BALB/c mice received a subcutaneous injection of 5 × 106 WEHI-p60 cells into the flank on day 0. Mice given the fibrosarcoma cells were inspected on days 4, 5, 7, 10 and 14 for tumor growth and size. a Immunization group A: application of Salmonella vaccine strain SB824 (pHR241) expressing translocated YopE/p60 via the intravenous route on day 0. b Immunization group B: application of Salmonella vaccine strain SB824 (pHR241) via the intravenous route on day 4. c Immunization group C: application of Salmonella vaccine strain SB824 (pHR241) via the orogastric route on day 0. d Immunization group D: application of Salmonella vaccine strain SB824 (pHR241) via the orogastric route on day 4. Negative control mice were immunized with plasmidless SB824 (S) or remained non-immunized (NI). Means and standard deviations of ten mice per vaccination group are indicated. Asterisks indicate values that differ significantly from the corresponding values obtained from mice of the control groups S and NI (*p < 0.01, **p < 0.005)
Mice of immunization group B received an intravenous vaccination with SB824 (pHR241) 4 days after tumor injection (Fig. 1b, black triangle). Control mice of immunization group S were immunized with plasmidless SB824 (Fig. 1b, black diamond) or remained non-immunized (NI) (Fig. 1b, black square). In Fig. 1b, it is shown that in mice vaccinated with SB824 (pHR241) peak fibrosarcoma growth on day 4 (45 ± 7 mm2) gradually declined on day 5 (29 ± 9 mm2) and day 7 (20 ± 8 mm2), and was significantly reduced on day 10 (10 ± 10 mm2) and on day 14 (9 ± 11 mm2). Interestingly, mice of the immunization group S revealed a transient tumor growth delay during the first days after vaccination. However, immunization with SB824 did not result in the reduction of WEHI-p60 growth on day 14 (63 ± 12 mm2) as compared to non-immunized mice (63 ± 9 mm2).
Mice of immunization groups C and D were orogastrically vaccinated with recombinant Salmonella. Animals of the immunization group C obtained vaccination with SB824 (pHR241) on day 0 simultaneously with tumor injection (Fig. 1c, black triangle). Control mice of the immunization group S were immunized with plasmidless SB824 (Fig. 1c, black diamond) or remained non-immunized (NI) (Fig. 1c, black square). Figure 1c reveals that in mice vaccinated with SB824 (pHR241), peak fibrosarcoma growth on day 5 (56 ± 11 mm2) was significantly reduced on day 10 (23 ± 10 mm2) and on day 14 (18 ± 9 mm2) after tumor injection. Interestingly, mice of the immunization group S revealed a transient tumor growth delay during the first few days after vaccination. However, immunization with SB824 did not result in the reduction of WEHI-p60 growth on day 14 (63 ± 14 mm2) as compared to non-immunized mice (63 ± 9 mm2).
Mice of the immunization group D received an orogastric vaccination with SB824 (pHR241) 4 days after tumor injection (Fig. 1d, black triangle). Control mice of immunization group S were immunized with plasmidless SB824 (Fig. 1d, black diamond) or remained non-immunized (NI) (Fig. 1d, black square). In Fig. 1d, it is demonstrated that in mice vaccinated with SB824 (pHR241) peak fibrosarcoma growth on day 5 (58 ± 9 mm2) gradually declined on day 7 (42 ± 9 mm2) and day 10 (23 ± 10 mm2), and was significantly reduced on day 14 (20 ± 12 mm2). Interestingly, mice of immunization group S revealed a transient tumor growth delay during the first few days after vaccination. However, immunization with SB824 did not result in the reduction of WEHI-p60 growth on day 14 (58 ± 7 mm2) as compared to non-immunized mice (63 ± 9 mm2).
Taken together, all four immunization strategies (A–D) using SB824 (pHR241) as a vaccine strain resulted in a highly significant reduction of fibrosarcoma size as compared to control mice.
Determination of the rate of tumor-free mice after intravenous or orogastric vaccination with recombinant Salmonella
In our previous study, we demonstrated in a prophylactic anti-tumor immunization setting that 80% of all tumor-challenged mice remained free of fibrosarcoma growth [27]. Gray bars in Fig. 2 reveal the percentages of tumor-free mice after intravenous or orogastric therapeutic vaccination with recombinant Salmonella. Determination of tumor growth was performed 14 days after WEHI-p60 inoculation. All mice from immunization groups A–D vaccinated with SB824 (pHR241) showed significantly higher tumor regression rates as compared to mice vaccinated with SB824 (immunization group S, 5 ± 4%) or to mice that remained non-immunized (immunization group NI, 4 ± 2%). While 71 ± 4% of mice from the immunization group A showed a complete regression of tumor growth, mice from immunization group B revealed an even higher regression rate (80 ± 2%). In contrast to intravenous vaccination with Salmonella, orogastric immunization led to significantly lower (**p < 0.005) protection rates (immunization group C, 52 ± 4%; immunization group D, 50 ± 5%). No significant differences could be detected between immunization groups A and B or C and D, respectively.
Percentages of tumor-free mice after intravenous or orogastric therapeutic vaccination with recombinant Salmonella. Determination of tumor size was performed 14 days after WEHI-p60 inoculation. Complete tumor regression was defined by a calliper detection threshold of 3 mm × 3 mm, successful protection against tumor rechallenge after 1 month and no cancer relapse during the observation period of 3 months. Immunization group A: application of Salmonella vaccine strain SB824 (pHR241) via the intravenous route on day 0. Immunization group B: application of SB824 (pHR241) via the intravenous route on day 4. Immunization group C: application of SB824 (pHR241) via the orogastric route on day 0. Immunization group D: application of SB824 (pHR241) via the orogastric route on day 4. Negative control mice were immunized with plasmidless SB824 (S) or remained non-immunized (NI). Means and standard deviations of ten mice per vaccination group are indicated. Asterisks indicate values that differ significantly from the corresponding values obtained from mice of control groups S and NI (***p < 0.001) or indicate values of groups A and B that differ significantly from those of groups C and D (**p < 0.005)
Co-staining of p60217–225-specific CD8 T cells with CD62L and CD127
We assumed that regression of fibrosarcoma growth was mediated by p60217–225-specific CD8 induced by vaccination with SB824 (pHR241). Antigen-specific T cells can be divided at least into three subsets [36]. Effector CD8 T cells (TEC) are characterized by an immediate effector function, poor proliferative activity and a limited in vivo survival. In contrast, memory T cells persist for extended periods due to an antigen-independent homeostatic turnover. Central memory T cells (TCMC) reside preferentially in lymphoid organs and lack immediate effector functions [20], whereas effector memory T cells (TEMC) migrate mainly into non-lymphoid tissue and elicit immediate effector functions on antigen reencounter. Recent results indicate that interleukin-7 receptor α-chain (CD127) surface expression is a marker for long-living memory T cells [15]. The combination of surface staining for CD127 and l-selectin (CD62L) further separates between TCMC (CD127high/CD62Lhigh) and TEMC (CD127high/CD62Llow) allowing to distinguish TEC (CD127low/CD62Llow) from memory T cells at early time points of in vivo immune responses.
To demonstrate that both long- and short-living CD8 T cells with an immediate effector function are induced by our therapeutic immunization strategies using recombinant Salmonella, blood and tumor samples from immunized mice were collected 14 days after tumor injection and p60217–225 tetramer-positive CD8 T cells were analyzed by flow cytometry (Figs. 3, 4). Our data show that p60-specific CD8 T cells could barely be detected in blood samples of mice vaccinated with plasmidless SB824 (Fig. 3, panel S). In contrast, intravenous or orogastric immunization with SB824 (pHR241) expressing translocated YopE/p60 resulted in a pronounced stimulation of p60217–225 tetramer-positive CD8 T cells in blood samples (Fig. 3, panels A and C) or fibrosarcoma tissue (Fig. 3, panel F). Furthermore, Fig. 5 reveals that vaccination with SB824 (pHR241) (immunization groups A–D) induces significantly higher absolute numbers of p60-specific CD8 T cells (500–2,000 per ml blood) as compared to immunization with plasmidless SB824 (38 ± 17 p60-specific CD8 T cells/ml blood, S) or non-immunized mice (42 ± 9 p60-specific CD8 T cells/ml blood, NI). The comparison of antigen-specific CD8 T cell responses in mice after intravenous vaccination (Fig. 5a, b) with mice after orogastric immunization (Fig. 5c, d) demonstrates that significantly higher absolute numbers of p60217–225 tetramer-positive CD8 T cells were found in blood samples from mice of immunization groups A and B (1,487 ± 176 and 1,928 ± 179 p60-specific CD8 T cells/ml blood) than in mice from groups C and D (524 ± 65 and 652 ± 63 p60-specific CD8 T cells/ml blood). These results are in line with our above described observations (Fig. 2) that intravenous vaccination with Salmonella leads to significant higher tumor regression rates than orogastric vaccination. Further analysis of lymphocytes from blood samples revealed a predominance of CD8 T cells with a CD62Llow-phenotype (Fig. 5). Thus, both TEMC and TEC constitute the majority of p60-specific lymphocytes in this non-lymphoid compartment, whereas relatively low levels of TCMC were detected.
Representative dot plots of p60217–225-tetramer-positive CD8 T cells in blood samples (panels A, C, and S) or tumor tissue (panel F) from BALB/c mice (n = 5 per group) determined 2 weeks after WEHI-p60 fibrosarcoma inoculation. Panels A and F show representative dot plots of mice immunized intravenously with SB824 (pHR241); panel C shows a representative dot plot of mice vaccinated with SB824 (pHR241) via the orogastric route. Panel S depicts a representative dot plot of mice vaccinated intravenously with plasmidless SB824
Differentiation patterns of p60-specific CD8 T cells into phenotypically distinct subsets in blood samples (panel A) or tumor tissue (panel F) from BALB/c mice (n = 5 per group) determined 2 weeks after WEHI-p60 fibrosarcoma inoculation and intravenous vaccination with SB824 (pHR241). Cells were stained for expression of CD127 (x-axis) and CD62L (y-axis). The percentage of cells in each quadrant is indicated. CD127high/CD62Lhigh central memory CD8 T cells (TCMC); CD127high/CD62Llow effector memory CD8 T cells (TEMC); CD127low/CD62Llow effector CD8 T cells (TEC)
Absolute numbers of p60-specific CD8 T cells per milliliter blood and differentiation patterns of phenotypically distinct subsets (TEMC, TEC and TCMC) on day 14 after tumor inoculation. Cells were stained for expression of CD127 and CD62L. Immunization group A: application of Salmonella vaccine strain SB824 (pHR241) via the intravenous route on day 0. Immunization group B: application of SB824 (pHR241) via the intravenous route on day 4. Immunization group C: application of SB824 (pHR241) via the orogastric route on day 0. Immunization group D: application of SB824 (pHR241) via the orogastric route on day 4. Immunization group S: application of SB824 via the intravenous route on day 0. NI: non-immunized mice received phosphate-buffered saline on day 0. Asterisks indicate values that differ significantly from the corresponding values obtained from mice of the control groups S and NI (***p < 0,001) or indicate values of groups A and B that differ significantly from those of group C and D (**p < 0.005)
Detection of p60-specific CD8 T cells in fibrosarcoma tissue
Immune cells such as antigen-specific CD8 T cells enter the skin and the subcutaneous compartment via the blood stream. As shown above, we analyzed the distribution of TEC, TCMC and TEMC subpopulations in peripheral blood (Fig. 5). We found that the majority of p60-specific lymphocytes belonged to the TEC and the TEMC CD8 T cell subsets. As recently demonstrated, WEHI-p60 cells are recognized in vitro by p60-specific CD8 T cells [27], so we hypothesized this to happen in vivo as well. Most likely, TEC and TEMC are responsible for the in vivo destruction and regression of inoculated WEHI-p60 cancer cells in our tumor model. To determine whether tumor regression was associated with the presence of p60-specific TEC and TEMC, fibrosarcoma tissue of mice from immunization group A underwent FACS analysis and tetramer staining. As shown in Fig. 6, we plotted WEHI-p60 growth against the numbers of CD8 T cells (light gray area) and p60217–225 tetramer-positive TEC and TEMC (dark gray area), respectively, per 103 tumor cells. Interestingly, CD8 T cells are already detectable in the tumor tissue on day 5 (0.026 ± 0/103 tumor cells). At this time point, no p60-specific CD8 T cells could be detected. However, on day 10 after fibrosarcoma challenge, p60217–225 tetramer-positive TEC and TEMC (0.0648 ± 0.046/103 tumor cells) were found in cancer tissue culminating 4 days later in a tenfold higher concentration (0.612 ± 0.072/103 tumor cells).
Detection of tumor-invading p60-specific CD8 T cells in fibrosarcoma tissue. Regression of tumor growth in mice intravenously vaccinated on day 0 with SB824 (pHR241) (see Fig. 1a) was plotted against the numbers of CD8 T cells (light gray area) and p60217-225 tetramer-positive TEC and TEMC (dark gray area), respectively, per 103 tumor cells. Means and standard deviations of ten mice per vaccination group are indicated
In summary, these results strongly support the hypothesis that tumor-invading p60-specific effector T cells contribute to the destruction of WEHI-p60 cells and the regression of this solid tumor.
Discussion
This study demonstrates that recombinant Salmonella can lead to a pronounced antigen-specific effector CD8 T cell induction, resulting in tumor regression in mice therapeutically immunized via the intravenous or the orogastric route. It is also shown that these effector lymphocytes accumulate in the regressing tumor tissue. Furthermore, we found that intravenous immunization induces significantly higher antigen-specific CD8 T cell frequencies and tumor regression rates as compared to the orogastric immunization route.
Anti-tumor vaccines are able to successfully promote tumor-specific T cell responses in both preclinical and clinical studies [25, 38]. However, induction of antitumor T cell immunity rarely results in an effective eradication of established diseases in murine models or patients [31]. It is well-established that CD8 T cells play a central role in the host defense against tumors [14]. However, the induction of antigen-specific effector CD8 T cell responses to fight cancer remains a major challenge in vaccine development. Live attenuated bacteria (e.g., Salmonella enterica, Listeria monocytogenes and Mycobacteria) have been widely used as vaccine carriers for foreign antigen display to induce cell-mediated immunity [7, 17]. In the past, the T3SS of S. enterica serovar Typhimurium became an attractive tool for heterologous protein delivery directly into the cytosol of macrophages and dendritic cells resulting in vigorous antigen-specific CD8 T cell priming and the induction of protective immunity against viruses, bacteria and tumors [27, 32, 34, 40]. Previously, we have demonstrated that a T3SS effector molecule from Yersinia can be used as a carrier protein for translocation of the large Listeria-derived p60 antigen by Salmonella’s T3SS [33]. After a single oral immunization of mice with recombinant Salmonella expressing translocated YopE/p60, the efficient stimulation of p60 peptide-specific CD8 T cells led to protection against listeriosis. Using this model for prophylactic anti-cancer therapy, we demonstrated that 80% of all Salmonella-vaccinated mice were protected against a subcutaneous challenge with a progressively grown fibrosarcoma transfected with p60. Furthermore, the remaining 20% of mice showed significant reduction in the development of tumor size as compared to control groups [27].
Based on these findings, we became interested in the model’s potential for therapeutic anti-cancer intervention. The idea of using bacteria as an anti-cancer treatment is quite old. William Coley described already in 1893 a transient regression of solid, especially soft tissue tumors after treatment with Gram-negative bacteria [5]. Today, we know that the lipopolysaccharides on the Salmonella surface enable CD14+ monocytes to secrete TNF, IL-1 and IL-6, which could explain the temporary tumor regression or growth delay during the first few days after Salmonella vaccination in our vaccination setting [30, 42]. Except or Bacille Calmette-Guérin (BCG) instillation in the treatment of superficial bladder cancer [24], to date the use of bacteria in anti-cancer therapy has shown limited success.
In the majority of tumor vaccination studies, post-therapy antitumor activity is assessed by monitoring peripheral T cell immunity [40]. This strategy provides a convenient and accurate method for quantification of tumor-specific T cells. However, it does not predict whether these cells will maintain effector activity once they encounter the highly immune-suppressive tumor milieu characterized by inhibitory cytokines, enzymes, death receptor ligands and T suppressor cells [23, 35, 41]. Probably, one of the best documented and most crucial mechanisms of cancer escape is the total or partial loss of MHC class I molecules in tumor cells, resulting in an insufficient presentation of antigenic peptides to the immune system [9, 10]. Further studies are needed to investigate whether genetically manipulated Salmonella vaccine strains can influence the immune-suppressive microenvironment of tumors.
Mainly, three criteria are required for destruction of tumor cells by the immune system [18]: first of all, sufficient numbers of naïve immune cells with highly avid recognition of tumor antigens must be generated in vivo; secondly, these antigen-specific cells must proliferate, survive and migrate to the tumor; and thirdly, especially in the case of solid tumors, they must infiltrate the tumor tissue to destroy the cancer cells efficiently. What subpopulation of tetramer-positive p60217–225-specific CD8 T cells conferred protection against WEHI-p60 fibrosarcoma cells in our experimental setup? It has been demonstrated that in the murine Listeria infection model, TEMC are the most effective CD8 T cell subsets conferring protection against this intracellular bacterium [1, 16]. Furthermore, the relationship between tumor-infiltrating T cells and improved clinical outcome and survival has been shown in various studies [4, 6, 26, 29, 43]. Since p60217–225-tetramer-positive TEC and TEMC represented the majority of p60-specific CD8 T cells in the peripheral blood of mice from all immunization groups vaccinated with SB824 (pHR241), it was tempting to speculate that these lymphocyte subsets were also present in the fibrosarcoma tissue. Even though it remains unclear whether a more rapid or a more effective induction of effector CD8 T cells caused the more successful outcome of the intravenous immunization route, a clearly positive correlation (τ = 0.666) between the amount of effector T cells in blood (Fig. 5) and the probability of tumor regression (Fig. 2) could be proven. Additionally, a pronounced accumulation of antigen-specific effector T cells was detected in the fibrosarcoma tissue (Figs. 3f, 4f) representing up to a third of all tumor-invading CD8 T cells correlating with the kinetics of tumor regression (Fig. 6).
The data presented in this report clearly demonstrate that our vaccination approach using T3SS-mediated antigen delivery by Salmonella is a very promising tool for future anti-cancer therapies. Recombinant Salmonella is able to induce sufficient amounts of p60-specific CD8 T cells in blood. These lymphocytes can migrate to the tumor, invade the tumor tissue and, according to the observed tumor regression and former in vitro experiments [27], most likely destroy the tumor cells. Further studies are needed to determine whether this vaccination approach can also be successful against cancer cells expressing naturally occurring tumor-associated antigens. It will be exciting to investigate if Salmonella may fulfill the promise suggested by the data presented in this proof-of-principle report.
References
Berchtold C, Panthel K, Jellbauer S, Köhn B, Roider E, Partilla M, Heesemann J, Endres S, Bourquin C, Rüssmann H (2009) Superior protective immunity against murine listeriosis by combined vaccination with CpG DNA and recombinant Salmonella. Infect Immun 77:5501–5508
Busch DH, Pilip I, Pamer EG (1998) Evolution of a complex TCR repertoire during primary and recall bacterial infection. J Exp Med 188:61–70
Busch DH, Pilip IM, Vijh S, Pamer EG (1998) Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353–362
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77:1303–1310
Coley WB (1893) The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin Orthop Relat Res 262:3–11
Diederichsen AC, Hjelmborg JB, Christensen PB, Zeuthen J, Fenger C (2003) Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother 52:423–428
Dougan G (1994) The molecular basis for the virulence of bacterial pathogens: implications for oral vaccine development. Microbiology 140:215–224
Galán JE (2001) Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol 17:53–86
Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern PL (1993) Natural history of HLA expression during tumour development. Immunol Today 10:491–499
Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL (1997) Implications for immunosurveillance of altered HLA class I phenotypes in human tumors. Immunol Today 18:89–95
Goedegebuure PS, Eberlein TJ (1995) The role of CD4+ tumor-infiltrating lymphocytes in human solid tumors. Immunol Res 14:119–131
Guard-Petter J (1998) Variants of smooth Salmonella enterica serovar Enteritidis that grow to higher cell density than the wild type are more virulent. Appl Environ Microbiol 6:2166–2172
Guttinger M, Guidi F, Chinol M, Reali E, Veglia F, Viale G, Paganelli G, Corti A, Siccardi AG (2000) Adoptive immunotherapy by avidin-driven cytotoxic T lymphocyte-tumor bridging. Cancer Res 60:4211–4215
Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM (2000) Eradication of established tumors by CD8+ T cells adoptive immunotherapy. Immunity 13:265–276
Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH (2004) Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA 101:5610–5615
Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH (2006) Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur J Immunol 36:1453–1464
Kotton CN, Hohmann EL (2004) Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect Immun 72:5535–5547
Lizée G, Cantu MA, Hwu P (2007) Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res 13:5250–5255
Marzo AL, Lake RA, Robinson BW, Scott B (1999) T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res 59:1071–1079
Masopust D, Vezys V, Marzo AL, Lefrancois L (2001) Preferential localization of effector memory cells in non-lymphoid tissue. Science 291:2413–2417
Medina E, Guzmán CA (2001) Use of bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 19:1573–1580
Minev BB, Mc Farland BJ, Spiess OJ, Rosenberg SA, Restifo NP (1994) Insertion signal sequence fused to minimal peptides elicits specific CD8+ T-cell responses and prolongs survival of thymoma-bearing mice. Cancer Res 54:4155–4161
Monsurro V, Wang E, Panelli MC, Nagorsen D, Jin P, Katia Z, Smith K, Ngalame Y, Even J, Marincola FM (2003) Active-specific immunization against melanoma: is the problem at the receiving end? Semin Cancer Biol 13:473–480
Morales A, Eidinger D, Bruce AW (1976) Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 116:180–183
Morse MA, Chui S, Hobeika A, Lyerly HK, Clay T (2004) Recent developments in therapeutic cancer vaccines. Nat Clin Pract Oncol 2:108–113
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Panthel K, Meinel KM, Sevil Domenech VE, Geginat G, Linkemann K, Busch DH, Rüssmann H (2006) Prophylactic anti-tumor immunity against a murine fibrosarcoma triggered by the Salmonella type III secretion system. Microbes Infect 8:2539–2546
Panthel K, Meinel KM, Sevil Domènech VE, Trülzsch K, Rüssmann H (2008) Salmonella type III-mediated antigen delivery: a versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int J Med Microbiol 298:99–103
Prall F, Dührkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M (2004) Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol 35:808–816
Proskuryakov SY, Gabai VL (2010) Mechanisms of tumor cell necrosis. Curr Pharm Des 16:56–68
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909–915
Rüssmann H, Shams H, Poblete F, Fu Y, Galán JE, Donis RO (1998) Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281:565–568
Rüssmann H, Igwe EI, Sauer J, Hardt WD, Bubert A, Geginat G (2001) Protection against murine listeriosis by oral vaccination with recombinant Salmonella expressing hybrid Yersinia type III proteins. J Immunol 167:357–365
Rüssmann H (2003) Bacterial type III translocation: a unique mechanism for cytosolic display of heterologous antigens by attenuated Salmonella. Int J Med Microbiol 293:107–112
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538–18543
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712
Seliger B, Maeurer MJ, Ferrone S (1997) TAP off—tumors on. Immunol Today 18:292–299
Slingluff CL Jr, Speiser DE (2005) Progress and controversies in developing cancer vaccines. J Transl Med 3:18
Staveley-O`Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H (1998) Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA 95:1178–1183
Walker EB, Disis ML (2003) Monitoring immune responses in cancer patients receiving tumor vaccines. Int Rev Immunol 22:283–319
Whiteside TL (2006) Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 16:3–15
Wright SD, Ramos RA, Patel M, Miller DS (1992) Septin: a factor in plasma that opsonizes lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med 176:719–727
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Acknowledgments
This research was conducted by Elisabeth Roider in partial fulfillment of the requirements for a Ph.D. from the Ludwig-Maximilians-University, Munich, Germany. Elisabeth Roider was supported by the “Förderprogramm für Forschung und Lehre” from the Ludwig-Maximilians-University Munich. Holger Rüssmann was supported by the Deutsche Forschungsgemeinschaft (grant RU 838/1-3 and grant RU 838/2-1). Christina Berchtold was supported by the Deutsche Forschungsgemeinschaft (Graduiertenkolleg 1202 “Oligonucleotides in Cell Biology and Therapy”). Dirk H. Busch was supported by the SFB 576 (TP-A8), SFB 456 (TP-B13) and TR-SFB 36 (TP-B10/13).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roider, E., Jellbauer, S., Köhn, B. et al. Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer. Cancer Immunol Immunother 60, 371–380 (2011). https://doi.org/10.1007/s00262-010-0950-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0950-x