Abstract
Background
The transcription factor, WT1, is highly overexpressed in malignant pleural mesothelioma (MPM) and immunohistochemical stains for WT1 are used routinely to aid in its diagnosis. Using computer prediction analysis we designed analog peptides derived from WT1 sequences by substituting amino acids at key HLA-A0201 binding positions. We tested the safety and immunogenicity of a WT1 vaccine comprised of four class I and class II peptides in patients with thoracic neoplasms expressing WT1.
Methods
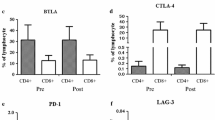
Therapy consisted of six subcutaneous vaccinations administered with Montanide adjuvant on weeks 0, 4, 6, 8, 10, and 12, with 6 additional monthly injections for responding patients. Injection sites were pre-stimulated with GM-CSF (70 mcg). Immune responses were evaluated by DTH, CD4 T-cell proliferation, CD8 T-cell interferon gamma release, intracellular cytokine staining, WT1 peptide MHC-tetramer staining, and cytotoxicity against WT1 positive tumor cells.
Results
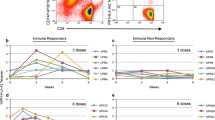
Nine patients with MPM and 3 with NSCLC were vaccinated, with 8 patients receiving at least 6 vaccinations; in total, 10 patients were evaluable for immune response. Six out of nine patients tested demonstrated CD4 T-cell proliferation to WT1 specific peptides, and five of the six HLA-A0201 patients tested mounted a CD8 T-cell response. Stimulated T cells were capable of cytotoxicity against WT-1 positive cells. Vaccination also induced polyfunctional CD8 T cell responses.
Conclusions
This multivalent WT1 peptide analog vaccine induces immune responses in a high proportion of patients with thoracic malignancies with minimal toxicity. A randomized trial testing this vaccine as adjuvant therapy in MPM is planned.





Similar content being viewed by others
References
Keilholz U, Menssen HD, Gaiger A, Menke A, Oji Y, Oka Y, Scheibenbogen C, Stauss H, Thiel E, Sugiyama H (2005) Wilms’ tumour gene 1 (WT1) in human neoplasia. Leukemia 19:1318–1323
Oji Y, Ogawa H, Tamaki H, Oka Y, Tsuboi A, Kim EH, Soma T, Tatekawa T, Kawakami M, Asada M, Kishimoto T, Sugiyama H (1999) Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res 90:194–204
Inoue K, Ogawa H, Sonoda Y, Kimura T, Sakabe H, Oka Y, Miyake S, Tamaki H, Oji Y, Yamagami T, Tatekawa T, Soma T, Kishimoto T, Sugiyama H (1997) Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood 89:1405–1412
Rosenfeld C, Cheever MA, Gaiger A (2003) WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia 17:1301–1312
Amin KM, Litzky LA, Smythe WR, Mooney AM, Morris JM, Mews DJ, Pass HI, Kari C, Rodeck U, Rauscher FJ 3rd et al (1995) Wilms’ tumor 1 susceptibility (WT1) gene products are selectively expressed in malignant mesothelioma. Am J Pathol 146:344–356
Scharnhorst V, Dekker P, van der Eb AJ, Jochemsen AG (1999) Internal translation initiation generates novel WT1 protein isoforms with distinct biological properties. J Biol Chem 274:23456–23462
Haber DA, Sohn RL, Buckler AJ, Pelletier J, Call KM, Housman DE (1991) Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci USA 88:9618–9622
Oates J, Edwards C (2000) HBME-1, MOC-31, WT1 and calretinin: an assessment of recently described markers for mesothelioma and adenocarcinoma. Histopathology 36:341–347
Foster MR, Johnson JE, Olson SJ, Allred DC (2001) Immunohistochemical analysis of nuclear versus cytoplasmic staining of WT1 in malignant mesotheliomas and primary pulmonary adenocarcinomas. Arch Pathol Lab Med 125:1316–1320
Ordonez NG (2003) The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 27:1031–1051
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15:5323–5337
Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, Elisseeva OA, Oji Y, Kawakami M, Ikegame K, Hosen N, Yoshihara S, Wu F, Fujiki F, Murakami M, Masuda T, Nishida S, Shirakata T, Nakatsuka S, Sasaki A, Udaka K, Dohy H, Aozasa K, Noguchi S, Kawase I, Sugiyama H (2004) Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA 101:13885–13890
Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, Hofmann WK, Uharek L, Thiel E, Scheibenbogen C (2009) A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 113:6541–6548
Pinilla-Ibarz J, May RJ, Korontsvit T, Gomez M, Kappel B, Zakhaleva V, Zhang RH, Scheinberg DA (2006) Improved human T-cell responses against synthetic HLA-0201 analog peptides derived from the WT1 oncoprotein. Leukemia 20:2025–2033
May RJ, Dao T, Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Zhang RH, Maslak P, Scheinberg DA (2007) Peptide epitopes from the Wilms’ tumor 1 oncoprotein stimulate CD4+ and CD8+ T cells that recognize and kill human malignant mesothelioma tumor cells. Clin Cancer Res 13:4547–4555
Yao TJ, Begg CB, Livingston PO (1996) Optimal sample size for a series of pilot trials of new agents. Biometrics 52:992–1001
Byrne MJ, Nowak AK (2004) Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 15:257–260
von Bergwelt-Baildon MS, Vonderheide RH, Maecker B et al (2002) Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 99:3319–3325
Seder RA, Darrah PA, Roederer M (2008) T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8:247–258
Perales MA, Yuan J, Powel S, Gallardo HF, Rasalan TS, Gonzalez C, Manukian G, Wang J, Zhang Y, Chapman PB, Krown SE, Livingston PO, Ejadi S, Panageas KS, Engelhorn ME, Terzulli SL, Houghton AN, Wolchok JD (2008) Phase I/II study of GM-CSF DNA as an adjuvant for a multipeptide cancer vaccine in patients with advanced melanoma. Mol Ther 16:2022–2029
Letsch A, Elisseeva OA, Scheibenbogen C, Asemissen A, Stather D, Busse A, Oka Y, Keilholz U, Sugiyama H, Thiel E (2008) Effect of vaccination of leukemia patients with a MHC class I peptide of Wilms tumor gene 1 (WT1) peptide with unspecific T helper stimulation on WT1-specific IgM responses and on IgG responses. J Clin Oncol 26:Abstr 3054
Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, Jafarpour B, Boss C, Barrett AJ (2008) Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood 111:236–242
Sandberg JK, Fast NM, Nixon DF (2001) Functional heterogeneity of cytokines and cytolytic effector molecules in human CD8+ T lymphocytes. J Immunol 167:181–187
Lichterfeld M, Yu XG, Waring MT, Mui SK, Johnston MN, Cohen D, Addo MM, Zaunders J, Alter G, Pae E, Strick D, Allen TM, Rosenberg ES, Walker BD, Altfeld M (2004) HIV-1-specific cytotoxicity is preferentially mediated by a subset of CD8(+) T cells producing both interferon-gamma and tumor necrosis factor-alpha. Blood 104:487–494
Krug LM, Pass HI, Rusch VW, Kindler HL, Sugarbaker DJ, Rosenzweig KE, Flores R, Friedberg JS, Pisters K, Monberg M, Obasaju CK, Vogelzang NJ (2009) Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 27:3007–3013
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G (2007) A clinical development paradigm for cancer vaccines and related biologics. J Immunother 30:1–15
Acknowledgments
We thank Dr. Bo Dupont and Alice Yeh at Memorial Sloan-Kettering Cancer Center for HLA genotyping and Dr. M. Roederer for providing the SPICE software. This study was supported by NIH PO1 23766, Innovive Pharmaceuticals, the Mesothelioma Applied Research Foundation, Tudor and Glades Funds and the Experimental Therapeutics Center of MSKCC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krug, L.M., Dao, T., Brown, A.B. et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol Immunother 59, 1467–1479 (2010). https://doi.org/10.1007/s00262-010-0871-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0871-8