Abstract
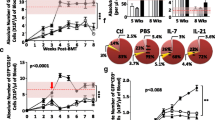
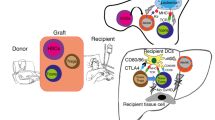
Thymic function decreases in line with tumor progression in patients with cancer, resulting in immunodeficiency and a poor prognosis. In the present study, we attempted to restore thymic function by BALB/c (H-2d) syngeneic (Syn), or B6 (H-2b) allogeneic (Allo) bone marrow transplantation (BMT) using intra-bone marrow–bone marrow transplantation (IBM–BMT) plus Syn-, Allo- or C3H (H-2k) 3rd-party fetal thymus transplantation (TT). Although the BALB/c mice with advanced tumors (Meth-A sarcoma; H-2d, >4 cm2) treated with either Syn- or Allo-BMT alone showed a slight improvement in survival compared with non-treated controls, the mice treated with BMT + TT showed a longer survival. The mice treated with Allo-BMT + Allo-TT or 3rd-party TT showed the longest survival. Interestingly, although there was no difference in main tumor size among the BMT groups, lung metastasis was significantly inhibited by Allo-BMT + Allo-TT or 3rd-party TT. Numbers of CD4+ and CD8+ T cells, Con A response, and IFN-γ production increased significantly, whereas number of Gr-1+/CD11b+ myeloid suppressor cells and the percentage of FoxP3+ cells in CD4+ T cells significantly decreased in these mice. Furthermore, there was a positive correlation between survival days and the number of T cells or T cell function, while there was a negative correlation between survival days and lung metastasis, the number of Gr-1+/CD11b+ cells, or the percentage of FoxP3+ cells. These results suggest that BMT + TT, particularly Allo-BMT + Allo-TT or 3rd-party TT, is most effective in prolonging survival as a result of the restoration of T cell function in hosts with advanced tumors.





Similar content being viewed by others
Abbreviations
- BMT:
-
Bone marrow transplantation
- IBM–BMT:
-
Intra-bone marrow–bone marrow transplantation
- TT:
-
Thymus transplantation
- BMC:
-
Bone marrow cell
- MHC:
-
Major histocompatibility complex
References
Toge T, Oride M, Yanagawa E et al (1982) Prognostic significance of lymphocyte proliferative responses to mitogens in gastric cancer patients. Jpn J Surg 12:424–428
Das SN, Khanna NN, Khanna S (1985) A multiparametric observation of immune competence in breast cancer and its correlation with tumour load and prognosis. Ann Acad Med Singapore 14:374–381
Adler A, Stein JA, Ben-Efraim S, Immunocompetence, immunosuppression, human breast cancer. III (1980) Prognostic significance of initial level of immunocompetence in early and advanced disease. Cancer 45:2074–2083
Adkins B, Charyulu V, Sun QL, Lobo D, Lopez DM (2000) Early block in maturation is associated with thymic involution in mammary tumor-bearing mice. J Immunol 164:5635–5640
Mandal D, Bhattacharyya A, Lahiry L, Choudhuri T, Sa G, Das T (2005) Failure in peripheral immuno-surveillance due to thymic atrophy: importance of thymocyte maturation and apoptosis in adult tumor-bearer. Life Sci 77:2703–2716
Carrio R, Lopez DM (2009) Impaired thymopoiesis occurring during the thymic involution of tumor-bearing mice is associated with a down-regulation of the antiapoptotic proteins Bcl-XL and A1. Int J Mol Med 23:89–98
Mandal D, Lahiry L, Bhattacharyya A, Bhattacharyya S, Sa G, Das T (2006) Tumor-induced thymic involution via inhibition of IL-7R alpha and its JAK-STAT signaling pathway: protection by black tea. Int Immunopharmacol 6:433–444
Bronte V, Apolloni E, Cabrelle A et al (2000) Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood 96:3838–3846
Kusmartsev SA, Li Y, Chen SH (2000) Gr-1 + myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol 165:779–785
Terabe M, Matsui S, Park JM et al (2003) Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med 98:1741–1752
Sakaguchi S, Ono M, Setoguchi R et al (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 212:8–27
Hoffmann P, Edinger M (2006) CD4+CD25+ regulatory T cells and graft-versus-host disease. Semin Hematol 43:62–69
Kono K, Kawaida H, Takahashi A et al (2006) CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 55:1064–1071
Wolf D, Wolf AM, Rumpold H et al (2005) The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11:8326–8331
Verdeguer A, Muñoz A, Cañete A et al (2004) Long-term results of high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma patients: a report of the Spanish working party for BMT in children (Getmon). Pediatr Hematol Oncol 21:495–504
Fefer A, Sullivan KM, Weiden P et al (1987) Graft versus leukemia effect in man: the relapse rate of acute leukemia is lower after allogeneic than after syngeneic marrow transplantation. Prog Clin Biol Res 244:401–408
Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG (2005) Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol 174:3051–3058
Kushida T, Inaba M, Hisha H et al (2001) Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood 97:3292–3299
Ikehara S (2008) A novel method of bone marrow transplantation (BMT) for intractable autoimmune diseases. J Autoimmun 30:108–113
Nakamura K, Inaba M, Sugiura K et al (2004) Enhancement of allogeneic hematopoietic stem cell engraftment and prevention of graft-versus-host diseases (GvHD) by intra-bone marrow–bone marrow transplantation plus donor lymphocyte infusion. Stem Cells 22:125–134
Hosaka N, Nose M, Kyogoku M, Nagata N et al (1996) Thymus transplantation, a critical factor for correction of autoimmune disease in aging MRL/+mice. Proc Natl Acad Sci USA 93:8558–8562
Hosaka N, Ryu T, Miyake T et al (2007) Treatment of autoimmune diseases in MRL/lpr mice by allogeneic bone marrow transplantation plus adult thymus transplantation. Clin Exp Immunol 147:555–663
Miyake T, Hosaka N, Cui W et al (2009) Adult thymus transplantation with allogeneic intra-bone marrow–bone marrow transplantation from same donor induces high thymopoiesis, mild graft-versus-host reaction and strong graft-versus-tumour effects. Immunology 126:552–564
Ryu T, Hosaka N, Miyake T et al (2008) Transplantation of newborn thymus plus hematopoietic stem cells can rescue supralethal irradiated mice. Bone Marrow Transplant 41:659–666
Nishida T, Hosaka N, Takaki T et al (2009) Allogeneic intra-BM–BMT plus adult thymus transplantation from same donor has benefits for long-term survival even after sublethal irradiation or low-dose BM cell injection. Bone Marrow Transplant 43:829–837
Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM (1999) Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med 341:1180–1189
Markert ML, Hicks CB, Bartlett JA, Harmon JL (2000) Effect of highly active antiretroviral therapy and thymic transplantation on immunoreconstitution in HIV infection. AIDS Res Hum Retroviruses 16:403–413
Cui W, Hosaka N, Miyake T et al (2008) Analysis of tolerance induction using triple chimeric mice: MHC-disparate thymus, hemopoietic cells and microenvironment. Transplantation 85:1151–1158
Edinger M, Hoffmann P, Ermann J et al (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 9:1144–1150
Scollay R, Jacobs S, Jerabek L, Butcher E, Weissman I (1980) T cell maturation: thymocyte and thymus migrant subpopulations defined with monoclonal antibodies to MHC region antigens. J Immunol 124:2845–2853
Lin YC, Chang LY, Huang CT, et al. Effector/memory but not naive regulatory T cells are responsible for the loss of concomitant tumor immunity. J Immunol 182:6095-6104
Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A (2003) CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98:1089–1099
Xiang X, Poliakov A, Liu C et al (2009) Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer 124:2621–2633
Hoechst B, Ormandy LA, Ballmaier M et al (2008) A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135:234–243
Ghiringhelli F, Puig PE, Roux S et al (2005) Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 202:919–929
Zhang L, Sun L, Zhao Y (2007) Thymic epithelial progenitor cells and thymus regeneration: an update. Cell Res 17:50–55
Takaki T, Hosaka N, Miyake T et al (2008) Presence of donor-derived thymic epithelial cells in [B6–>MRL/lpr] mice after allogeneic intra-bone marrow–bone marrow transplantation (IBM–BMT). J Autoimmun 31:408–415
Acknowledgments
This work was supported by a grant from Haiteku Research Center of the Ministry of Education, a grant from the Millennium Program of the Ministry of Education, Culture, Sports, Science, and Technology, a grant from the Science Frontier Program of the Ministry of Education, Culture, Sports, Science, and Technology, a grant from The 21st Century Center of Excellence (COE) Program of the Ministry of Education, Culture, Sports, Science, and Technology, a Research Grant B from Kansai Medical University, Health and Labor Sciences research grants (Research on Human Genome, Tissue Engineering Food Biotechnology), a grant from the Department of Transplantation for Regeneration Therapy (sponsored by Otsuka Pharmaceutical Co., Ltd.), a grant from the Molecular Medical Science Institute (Otsuka Pharmaceutical Co., Ltd.), and a grant from Japan Immunoresearch Laboratories Co., Ltd. (JIMRO). We wish to thank Ms. Y. Tokuyama, and Ms. A. Kitajima for their technical assistance, and Mr. Hilary Eastwick-Field, and Ms. K. Ando for their help in the preparation of the manuscript.
Conflict of interest statement
We have no conflict of interest in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Hosaka and W. Cui contributed equally to this study.
Rights and permissions
About this article
Cite this article
Hosaka, N., Cui, W., Zhang, Y. et al. Prolonged survival in mice with advanced tumors treated with syngeneic or allogeneic intra-bone marrow–bone marrow transplantation plus fetal thymus transplantation. Cancer Immunol Immunother 59, 1121–1130 (2010). https://doi.org/10.1007/s00262-010-0840-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0840-2