Abstract
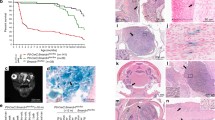
Inbred rat strains BDIX and BDIV are constitutionally susceptible and resistant, respectively, to the development of malignant peripheral nerve sheath tumors (MPNST) induced by neonatal exposure to N-ethyl-N-nitrosourea (EtNU). They represent a model system for analysis of molecular and cellular processes underlying differential cancer susceptibility. A point mutation in the Neu/ErbB-2 gene is an early marker of Schwann precursor cells at high risk of malignant conversion and is diagnostic of the resulting MPNST predominantly developing in the trigeminal nerves. Initially considerable amounts of Neu/ErbB-2-mutant cells arise in nerve tissue of both rat strains subsequently disappearing in resistant BDIV rats, but persisting and giving rise to MPNST in susceptible BDIX animals. An almost identical cellular immune response—sequentially involving macrophages, T helper- and cytotoxic T lymphocytes—is mounted in the trigeminal nerves of EtNU-treated rats of both strains. In this study, T cell maturation was prevented by neonatal thymectomy following EtNU-exposure. While resistance against MPNST development significantly decreased in BDIV rats MPNST incidence and survival time remained unaltered in thymectomized BDIX rats. Contrary to euthymic animals a number of both thymectomized BDIV and BDIX rats developed MPNST lacking the Neu/ErbB-2-mutation. This suggests that Schwann cells initiated by other genetic alterations can progress to full malignancy in immune-compromised rats only. T cell-dependent resistance against tumorigenesis originating from non-Neu/ErbB-2-mutant Schwann precursors might thus be shared by both strains while BDIV T lymphocytes additionally prevent the development of Neu/ErbB-2-mutant MPNST. Rat strain-specific differences in the interaction of T lymphocytes with (pre)malignant Neu-mutant cells may thus critically contribute to susceptibility and resistance towards EtNU-induced MPNST development.




Similar content being viewed by others
References
Balner H, Dersjant H (1966) Neonatal thymectomy and tumor induction with methylcholanthrene in mice. J Natl Cancer Inst 36:513
Bargmann CI, Hung MC, Weinberg RA (1986) Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell 45:649
Blankenstein T (2005) The role of tumor stroma in the interaction between tumor and immune system. Curr Opin Immunol 17:180
Bonnotte B, Larmonier N, Favre N et al (2001) Identification of tumor-infiltrating macrophages as the killers of tumor cells after immunization in a rat model system. J Immunol 167:5077
Burnet FM (1970) The concept of immunological surveillance. Prog Exp Tumor Res 13:1
Burstein NA, Law LW (1971) Neonatal thymectomy and non-viral mammary tumours in mice. Nature 231:450
Druckrey H (1971) Genotypes and phenotypes of ten inbred strains of BD-rats. Arzneimittelforschung 21:1274
Druckrey H, Landschutz C, Ivankovic S (1970) Transplacental induction of malignant tumours of the nervous system. II. Ethyl-nitrosurea in 10 genetically defined strains of rats. Z Krebsforsch 73:371
Druckrey H, Schagen B, Ivankovic S (1970) Induction of neurogenic malignancies by one single dose of ethyl-nitrosourea (ENU) given to newborn and juvenile BD IX-strain rats. Z Krebsforsch 74:141
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 3:991
Fieldsteel AH, Sato N, Colston MJ (1981) Relationship between T-cell population in neonatally thymectomized Lewis rats and susceptibility to infection with mycobacterium leprae. Int J Lepr Other Mycobact Dis 49:317
Gering KM, Marx JA, Lennartz K, Fischer C, Rajewsky MF, Kindler-Rohrborn A (2006) The interaction mode of premalignant Schwann and immune effector cells during chemically induced carcinogenesis in the rat peripheral nervous system is strongly influenced by genetic background. Cancer Res 66:4708
Gomory G (1937) Silver impregnation of reticulum in paraffin sections. Am J Pathol 13:993
Hunig T, Torres-Nagel N, Mehling B, Park HJ, Herrmann T (2001) Thymic development and repertoire selection: the rat perspective. Immunol Rev 184:7
Ivankovic S, Druckrey H (1968) Transplacental induction of malignant tumors of the nervous system. I. Ethyl-nitroso-urea (ENU) in BD IX rats. Z Krebsforsch 71:320
Johnson S (1968) The effect of thymectomy and of the dose of 3-methylcholanthrene on the induction and antigenic properties of sarcomas in C56Bl mice. Br J Cancer 22:93
Kindler-Rohrborn A, Kolsch BU, Fischer C, Held S, Rajewsky MF (1999) Ethylnitrosourea-induced development of malignant schwannomas in the rat: two distinct loci on chromosome of 10 involved in tumor susceptibility and oncogenesis. Cancer Res 59:1109
Kindler-Rohrborn A, Kind AB, Koelsch BU, Fischer C, Rajewsky MF (2000) Suppression of ethylnitrosourea-induced schwannoma development involves elimination of neu/erbB-2 mutant premalignant cells in the resistant BDIV rat strain. Cancer Res 60:4756
Koebel CM, Vermi W, Swann JB et al (2007) Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450:903
Koelsch BU, Fischer C, Neibecker M et al (2006) Gender-specific polygenic control of ethylnitrosourea-induced oncogenesis in the rat peripheral nervous system. Int J Cancer 118:108
Law LW (1966) Studies of thymic function with emphasis on the role of the thymus in oncogenesis. Cancer Res Cancer Res 26(4) 551–574
Maleckar JR, Sherman LA (1987) The composition of the T cell receptor repertoire in nude mice. J Immunol 138:3873
Muller R, Rajewsky MF (1983) Elimination of O6-ethylguanine from the DNA of brain, liver, and other rat tissues exposed to ethylnitrosourea at different stages of prenatal development. Cancer Res 43:2897
Nikitin A, Ballering LA, Lyons J, Rajewsky MF (1991) Early mutation of the neu (erbB-2) gene during ethylnitrosourea-induced oncogenesis in the rat Schwann cell lineage. Proc Natl Acad Sci USA 88:9939
Perantoni AO, Rice JM, Reed CD, Watatani M, Wenk ML (1987) Activated neu oncogene sequences in primary tumors of the peripheral nervous system induced in rats by transplacental exposure to ethylnitrosourea. Proc Natl Acad Sci USA 84:6317
Rajewsky MF, Augenlicht LH, Biessmann H et al (1977) Nervous-system-specific Carcinogenesis by ethylnitrosourea in the rat: molecular and cellular aspects. In: Hiatt HH, Watson JD, Winston JA (eds) Origins of human cancer. Cold Spring Harbor Laboratory, Cold Spring Harbor, pp 709
Rolstad B (2001) The athymic nude rat: an animal experimental model to reveal novel aspects of innate immune responses? Immunol Rev 184:136
Shankaran V, Ikeda H, Bruce AT et al (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410:1107
Stutman O (1975) Immunodepression and malignancy. Adv Cancer Res 22:261
Terszowski G, Muller SM, Bleul CC et al (2006) Evidence for a functional second thymus in mice. Science 312:284
Trainin N, Linker-Israeli M, Small M, Boiato-Chen L (1967) Enhancement of lung adenoma formation by neonatal thymectomy in mice treated with 7, 12-dimethylbenz(a)anthracene or urethan. Int J Cancer 2:326
Turusov VS, Cabral JP (1994) S-100 protein and glial fibrillary acidic protein (GFAP) in experimentally induced and spontaneous tumours of peripheral nerves in BDVI rats. Light microscopic and immunohistochemical study. Exp Toxicol Pathol 46:343
van den Broek ME, Kagi D, Ossendorp F et al (1996) Decreased tumor surveillance in perforin-deficient mice. J Exp Med 184:1781
Waynforth HB, Flecknell PA (1992) Experimental and surgical technique in the rat. In: Waynforth HB, Flecknell PA (eds) Experimental and surgical technique in the rat. Academic Press, San Diego, p 308
Willimsky G, Blankenstein T (2005) Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 437:141
Acknowledgments
The authors wish to thank Thomas Haberland and Michael Oepp at the Institute of Cell Biology (Cancer Research), University of Duisburg-Essen Medical School, for expert assistance with animal husbandry and surgical procedures. J. A. M. M was the recipient of a scholarship from the Graduate Program of the Deutsche Forschungsgemeinschaft, Bonn “Pathogenesis of nervous system diseases” which also provided funds for this work together with the Bonfor-Program (University of Bonn Research Council).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marx, J.A.M., Röhrborn, A.J., Koelsch, B.U. et al. Ablation of T cell immunity differentially influences tumor risk in inbred BD rat strains. Cancer Immunol Immunother 58, 1287–1295 (2009). https://doi.org/10.1007/s00262-008-0641-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-008-0641-z