Abstract
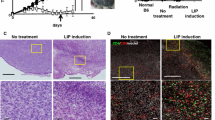
Previous studies by others using transplantable murine tumor models have demonstrated that the administration of antibodies that block CTLA-4 interaction with B7 can provoke the elimination of established tumors, and that the tumor suppression is mediated by T-cells and/or cells expressing NK1.1. Studies from our lab have established in a human/severe combined immunodeficient (SCID) mouse chimeric model that autologous peripheral blood leukocytes (PBL) can suppress the growth of tumor xenografts in a PBL dose-dependent fashion, and that this suppression is dependent upon the patient’s T and NK cells. Using this human/mouse chimeric model, we sought to determine whether an antibody blockade of CTLA-4 would enhance the anti-tumor response of a patient’s PBL. It was first important to determine whether the tumor suppression observed in the SCID model was dependent upon CD28/B7 co-stimulation. Blockade of B7 with a human CTLA-4-Ig fusion protein completely abrogated the lymphocyte-mediated tumor suppression, confirming in this model that tumor suppression is dependent upon a CD28/B7 co-stimulation. Using two different CTLA-4 specific monoclonal antibodies, we observed that CTLA-4 blockade significantly enhanced the human lymphocyte-mediated tumor suppression in mice co-engrafted with PBL and tumor cells. This enhancement was observed in both an allogeneic setting (in which the PBL were allogeneic with respect to the tumor) and an autologous setting (in which the PBL and tumor were from the same patient). These results sustain the notion that human anti-tumor immune response can be augmented (in vivo) by blocking the interaction between CTLA-4 and B7.





Similar content being viewed by others
Abbreviations
- CTLA-4:
-
Cytotoxic T Lymphocyte associated Antigen 4
- SCID:
-
Severe combined immunodeficient
- MLR:
-
Mixed lymphocyte reaction
- PBL:
-
Peripheral blood leukocytes
- DC:
-
Dendritic cells
- NK:
-
Natural killer cells
References
Boon T, van der Bruggen P (1996) Human tumor antigens recognized by T lymphocytes. J Exp Med 183:725–729
Rosenberg SA (1999) A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 10:281–287
Cohen PA, Peng L, Plautz GE, et al. (2000) CD4+ T cells in adoptive immunotherapy and the indirect mechanism of tumor rejection. Critical Reviews in Immunology 20:17–56
Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S (2000) Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol 74:181–273
Pardoll D (2003) Does the immune system see tumors as foreign or self? Annu Rev Immunol 21:807–839
Perez-Diaz A, Spiess PJ, Restifo NP, et al. (2002) Intensity of the vaccine-elicited immune response determines tumor clearance. J Immunol 168:338–347
Chambers CA, Allison JP (1997) Co-stimulation in T cell responses. Curr Opin Immunol 9:396–404
Mueller DL, Jenkins MK, Schwartz RH (1989) Clonal expansion versus functional clonal inactivation: A costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol 7:445–480
Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Immunol 2:116–126
Brunet JF, Denizot F, Luciani MF, et al (1987) A new member of the immunoglobulin superfamily- CTLA-4. Nature 328:267–270
Linsley PS, Brady W, Urnes M, et al. (1991) CTLA-4 is a second receptor for the B-cell activation antigen B7. J Exp Med 174:561–569
Linsley PS, Greene JL, Brady W, et al (1994) Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1:793–801
van der Merwe PA, Bodian DL, Daenke S, et al. (1997) CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med 185:393–403
Walunas TL, Lenschow DJ, Bakker CY, et al. (1994) CLTA-4 can function as a negative regulator of T cell activation. Immunity 1:405–413
Krummel MF, Allison JP (1995) CD28 and CTLA-4 have opposing effects on the response of T-cells to stimulation. J Exp Med 182:459–465
Waterhouse P, Penninger JM, Timms E, et al. (1995) CTLA-4 deficiency causes lymphoproliferative disorder with early lethality. Science 1995; 270:985–988
Tivol EA, Borriello F, Schweitzer AN, et al. (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role for CLTA-4. Immunity 3:541–547
Egen JG, Kuhns MS, Allison JP. (2002) CLTA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 3:611–618
Lee KH, Holdorf AD, Dustin ML, et al. (2002) T cell receptor signaling precedes immunological synapse formation. Science 295:1539–1542
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271:1734–1736
van Elsas A, Hurwitz AA, Allison JP (1999) Combination immunotherapy of B16 melanoma using anti-CTLA-4 and GM-CSF producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190:355–366
Bankert RB, Egilmez NK, Hess SD (2001) Human/SCID chimeric models for preclinical evaluation of anti-cancer therapies: Applications, limitations and future directions. Trends Immunol 22:368–393
Egilmez NK, Hess SD, Chen FA, et al. (2002) CD4+ effector T-cells mediate an indirect IL-12 and IFN-gamma-dependent suppression of autologous lung tumor xenografts in SCID mice. Cancer Res 62:2611–2617
Iwanuma Y, Chen FA, Egilmez NK, et al (1997) Antitumor immune response of human peripheral blood lymphocytes co-engrafted with tumor nito severe immunodeficient mice. Cancer Research 57:2937–2942
Linsley PS, Greene JL, Tan P, et al. (1992) Co-expression and functional cooperation of CTLA-4 and CD28 on activated T-lymphocytes. J Exp Med 176:1595–1604
Bender A, Sapp M, Schuler G, et al. (1996) Improved methods for the generation of dendritic cells from non-proliferating progenitors in human blood. J Immunol Methods 196:121–135
Mosmann T (1993) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Liu Y (1997) Is CTLA-4 a negative regulator for T-cell activation? Immunol Today 18:569–72
Thompson CB, Allison JP (1997) The emerging role of CTLA-4 as an immune attenuator. Immunity 7:445–450
Yang Y (1997) Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte associated molecule-4 blockade: The effect is manfiested only at the restricted tumor-bearing stages. Cancer Res 57:4036–4041
Hurwitz AA, Yu TF, Leach DR, Allison JP (1998) CLTA-4 blockade synergizes with tumor-derived GM-CSF for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA (10067–10071)
Hurwitz AA, Foster BA, Kwon ED, et al (2000) Combination immunotherapy of primary prostate cancer ina transgenic mouse model using CTLA-4 blockade. Cancer Res 60:2444–2448
Mokyr MB, Kalinchenko T, Gorelik L, Bluestone JA (1998) Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Research 58:5301–5304
Phan GQ, Yang JC, Sherry RM, et al (2003) Cancer regression and autoimmunity induced by cytotoxic T lymphocyte associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 100:8372–8377
Hodi FS, Mihm MC, Soiffer RJ, et al (2003) Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 100:4712–4717
Acknowledgments
The authors thank James P. Allison for reading the manuscript and providing critical comments and helpful suggestions. We greatly appreciate the help from Mr. Robert Parsons and the DLAR staff at RPCI for their help and support with the SCID mouse colony. We are grateful to Dr. Ron Gladue from Pfizer, Inc. for the gift of the P2 anti-CTLA-4 antibody and the isotype control antibody and to Dr. Robert Peach from Bristol-Myers Squibb who supplied us with the CTLA-4 Ig fusion protein, the L6 fusion protein control and the anti-CTLA-4 antibody 10A8. We thank Ms. Sandra Yokota and Ms. Jenni Loyall for their excellent technical support and Cheryl Zuber for helping with the preparation and typing of this manuscript.This work was supported in part by U.S. Public Health Service Grants CA79879 and CA96528 (to R.B.B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabel, M.S., Hess, S.D., Egilmez, N.K. et al. CTLA-4 blockade augments human T lymphocyte-mediated suppression of lung tumor xenografts in SCID mice. Cancer Immunol Immunother 54, 944–952 (2005). https://doi.org/10.1007/s00262-005-0668-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-005-0668-3