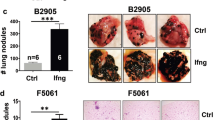
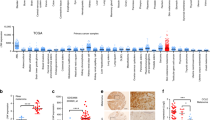
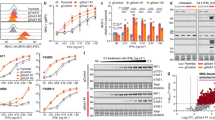
Abstract
Interferon-alpha (IFN-α) is used as an adjuvant therapy in patients with malignant melanoma and who have undergone surgical resection of high-risk lesions. Defective expression or activation of STAT1 or STAT2 has been shown to correlate with IFN-α or resistance in vitro; however, recent data from our laboratory suggest that the anti-tumor effects of IFN-α are dependent on STAT1 signaling within host immune cells. We measured STAT1 and STAT2 expression in 28 melanoma biopsies (8 cutaneous lesions; 1 lung metastasis; 19 nodal metastases) obtained from patients prior to the initiation of adjuvant IFN-α therapy. Disease recurrence following IFN-α treatment did not correlate with the staining intensity of either STAT1 (P=0.61) or STAT2 (P=0.52). Tumors with minimal STAT1 or STAT2 expression (<20% positive) were present in four patients with tumor-positive lymph nodes, who exhibited prolonged relapse-free survival (>44 months) following adjuvant therapy. Conversely, high levels of STAT1 were present in a patient who recurred during the course of IFN-α therapy. A case study of one patient who experienced recurrent disease during IFN-α treatment revealed that STAT1 levels were greater in the recurrent tumor when compared to the original lesion. These studies provide direct evidence to suggest that levels of STAT1 and STAT2 within the tumor do not influence a patient’s response to adjuvant IFN-α.




Similar content being viewed by others
Abbreviations
- IFN-α:
-
Interferon-alpha
- IFN-α2b:
-
Interferon-alpha 2b
- STAT:
-
Signal transducer and activator of transcription
- H & E:
-
Hematoxylin and eosin
References
Badgwell B, Lesinski GB, Magro C, Abood G, Skaf A, Carson WE (2003) The anti-tumor effects of interferon-alpha are maintained in mice challenged with a STAT1-deficient murine melanoma cell line. J Surg Res 116:129
Belardelli F, Ferrantini M, Proietti E, Kirkwood JM (2002) Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev 13:119
Carson WE (1998) Interferon-alpha-induced activation of signal transducer and activator of transcription proteins in malignant melanoma. Clin Cancer Res 4:2219
Creagan ET, Dalton RJ, Ahmann DL, Jung SH, Morton RF, Langdon RM Jr, Kugler J, Rodrigue LJ (1995) Randomized, surgical adjuvant clinical trial of recombinant interferon alfa-2a in selected patients with malignant melanoma. J Clin Oncol 13:2776
Chawla-Sarkar M, Leaman DW, Jacobs BS, Tuthill RJ, Chatterjee-Kishore M, Stark GR, Borden EC (2002) Resistance to interferons in melanoma cells does not correlate with the expression or activation of signal transducer and activator of transcription 1 (stat1). J Interferon Cytokine Res 22:603
Darnell JE Jr, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415
Hancock BW, Wheatley K, Harris S, Ives N, Harrison G, Horsman JM, Middleton MR, Thatcher N, Lorigan PC, Marsden JR, Burrows L, Gore M (2004) Adjuvant interferon in high-risk melanoma: the AIM HIGH Study–United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low-dose extended-duration interferon Alfa-2a in high-risk resected malignant melanoma. J Clin Oncol 22:53
Haque SJ, Williams BR (1998) Signal transduction in the interferon system. Semin Oncol 25:14
Jackson DP, Watling D, Rogers NC, Banks RE, Kerr IM, Selby PJ, Patel PM (2003) The JAK/STAT pathway is not sufficient to sustain the antiproliferative response in an interferon-resistant human melanoma cell line. Melanoma Res 13:219
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457
Kim SH, Cohen B, Novick D, Rubinstein M (1997) Mammalian type I interferon receptors consists of two subunits: IFNaR1 and IFNaR2. Gene 196:279
Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, Smith TJ, Rao U, Steele M, Blum RH (2000) High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 18:2444
Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U (2001) High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol 19:2370
Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U (2004) A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res 10:1670
Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH (1996) Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 14:7
Kovarik J, Boudny V, Kocak I, Lauerova L, Fait V, Vagundova M (2003) Malignant melanoma associates with deficient IFN-induced STAT 1 phosphorylation. Int J Mol Med 12:335
Lens MB, Dawes M (2002) Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol 20:1818
Leonard WJ, O’Shea JJ (1998) Jaks and STATs: biological implications. Annu Rev Immunol 16:293
Lesinski GB, Anghelina M, Zimmerer J, Bakalakos T, Badgwell B, Parihar R, Hu Y, Becknell B, Abood G, RayChaudhury A, Magro C, Durbin J, Carson WE (2003) The anti-tumor effects of interferon-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest 112:170
Lesinski GB, Kondadasula SV, Crespin T, Shen L, Kendra K, Walker MJ, Carson WE (2004) Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon alfa immunotherapy. J Natl Cancer Inst 96:1131
Levy DE, Gilliland DG (2000) Divergent roles of STAT1 and STAT5 in malignancy as revealed by gene disruptions in mice. Oncogene 19:2505
Pansky A, Hildebrand P, Fasler-Kan E, Baselgia L, Ketterer S, Beglinger C, Heim MH (2000) Defective Jak-STAT signal transduction pathway in melanoma cells resistant to growth inhibition by interferon-alpha. Int J Cancer 85:720
Rousseau DL Jr, Ross MI, Johnson MM, Prieto VG, Lee JE, Mansfield PF, Gershenwald JE (2003) Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol 10:569
Schneeberger A, Koszik F, Stingl G (1995) Immunologic host defense in melanoma: delineation of effector mechanisms involved and of strategies for the augmentation of their efficacy. J Invest Dermatol 105:110S
Sun WH, Pabon C, Alsayed Y, Huang PP, Jandeska S, Uddin S, Platanias LC, Rosen ST (1998) Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 91:570
Thompson JA (2002) The revised American Joint Committee on Cancer staging system for melanoma. Semin Oncol 29:361
Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H (2004) Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 10:48
Wong LH, Hatzinisiriou I, Devenish RJ, Ralph SJ (1998) IFN-gamma priming up-regulates IFNstimulated gene factor 3 (ISGF3) components, augmenting responsiveness of IFNresistant melanoma cells to type I IFNs. J Immunol 160:5475
Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ, Ralph SJ (1997) Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J Biol Chem 272:28779
Zhang YW, Wang LM, Jove R, Vande Woude GF (2002) Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 21:217
Acknowledgements
The authors thank Patrick Roche and The Ohio State University Histology Core Facility for assistance in optimizing immunohistochemical staining. We also thank Judith Bowers for her help in the management of clinical data. This work was supported by National Institutes of Health (NIH) Grants CA84402, P30-CA16058, The Valvano Foundation for Cancer Research Award, and The Ohio State University Department of Surgery Clinical Science Seed Grant. GBL is a NRSA T32 fellow (5 T32 CA90223-02). The Tissue Procurement Shared Resource of the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio is supported in part by NIH Grant P30 CA16059.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lesinski, G.B., Valentino, D., Hade, E.M. et al. Expression of STAT1 and STAT2 in malignant melanoma does not correlate with response to interferon-alpha adjuvant therapy. Cancer Immunol Immunother 54, 815–825 (2005). https://doi.org/10.1007/s00262-004-0649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0649-y