Abstract.
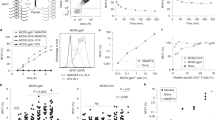
In this study, four modified gp100 peptides were designed by combining amino acids from the melanoma peptide antigen gp100(209–217) with preferred primary and auxiliary HLA-A *0201 anchor residues previously identified from combinatorial peptide library screening with recombinant HLA-A*0201. These modified peptides demonstrated stronger binding affinity for the HLA-A*0201 molecule compared to wild-type gp100 peptide. Nine CTL lines generated from patients immunized with the g209-2 M peptide and one CTL line from a non-immunized patient were tested for the ability to respond to these modified gp100 peptides. Stimulation of CTL by two of four modified peptides induced higher levels of IFN-γ secretion than the wild-type gp100 peptide, demonstrating that higher peptide binding affinity for HLA molecules does not necessarily equate to functional activity of CTL. Two major and one minor CTL recognition pattern were observed, irrespective of previous peptide immunization, suggesting that multiple, rationally designed modified tumor peptides for the same epitope stimulate a broad CTL response by activating multiple CTL capable of cross-reacting with the natural antigenic peptide.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Dionne, S.O., Smith, M.H., Marincola, F.M. et al. Functional characterization of CTL against gp100 altered peptide ligands. Cancer Immunol Immunother 52, 199–206 (2003). https://doi.org/10.1007/s00262-002-0358-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00262-002-0358-3