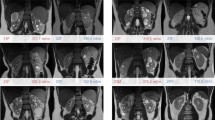
Abstract
Background and purpose
The objective is to demonstrate feasibility of quantitative susceptibility mapping (QSM) in autosomal dominant polycystic kidney disease (ADPKD) patients and to compare imaging findings with traditional T1/T2w magnetic resonance imaging (MRI).
Methods
Thirty-three consecutive patients (11 male, 22 female) diagnosed with ADPKD were initially selected. QSM images were reconstructed from the multiecho gradient echo data and compared to co-registered T2w, T1w, and CT images. Complex cysts were identified and classified into distinct subclasses based on their imaging features. Prevalence of each subclass was estimated.
Results
QSM visualized two renal calcifications measuring 9 and 10 mm and three pelvic phleboliths measuring 2 mm but missed 24 calcifications measuring 1 mm or less and 1 larger calcification at the edge of the field of view.
A total of 121 complex T1 hyperintense/T2 hypointense renal cysts were detected. 52 (43%) Cysts appeared hyperintense on QSM consistent with hemorrhage; 60 (49%) cysts were isointense with respect to simple cysts and normal kidney parenchyma, while the remaining 9 (7%) were hypointense. The presentation of the latter two complex cyst subtypes is likely indicative of proteinaceous composition without hemorrhage.
Conclusion
Our results indicate that QSM of ADPKD kidneys is possible and uniquely suited to detect large renal calculi without ionizing radiation and able to identify properties of complex cysts unattainable with traditional approaches.
Graphical abstract






Similar content being viewed by others
References
Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329(5):332–42. doi: https://doi.org/10.1056/NEJM199307293290508.
Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Abad JM, et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: prevalence and survival—an analysis of data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2014;29(Suppl 4):iv15–25. doi: https://doi.org/10.1093/ndt/gfu017.
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–301. doi: https://doi.org/10.1016/S0140-6736(07)60601-1.
Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38(4):777–84. doi: https://doi.org/10.1053/ajkd.2001.27720.
Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019;393(10174):919–35. doi: https://doi.org/10.1016/S0140-6736(18)32782-X.
Suwabe T, Ubara Y, Sumida K, Hayami N, Hiramatsu R, Yamanouchi M, et al. Clinical features of cyst infection and hemorrhage in ADPKD: new diagnostic criteria. Clin Exp Nephrol. 2012;16(6):892–902. doi: https://doi.org/10.1007/s10157-012-0650-2.
Riyahi S, Dev H, Blumenfeld JD, Rennert H, Yin X, Attari H, et al. Hemorrhagic Cysts and Other MR Biomarkers for Predicting Renal Dysfunction Progression in Autosomal Dominant Polycystic Kidney Disease. J Magn Reson Imaging. 2021;53(2):564–76. doi: https://doi.org/10.1002/jmri.27360.
Cornec-Le Gall E, Audrezet MP, Rousseau A, Hourmant M, Renaudineau E, Charasse C, et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2016;27(3):942–51. doi: https://doi.org/10.1681/ASN.2015010016.
Suwabe T, Ubara Y, Ueno T, Hayami N, Hoshino J, Imafuku A, et al. Intracystic magnetic resonance imaging in patients with autosomal dominant polycystic kidney disease: features of severe cyst infection in a case–control study. BMC Nephrol. 2016;17(1):170. doi: https://doi.org/10.1186/s12882-016-0381-9.
Marotti M, Hricak H, Fritzsche P, Crooks LE, Hedgcock MW, Tanagho EA. Complex and simple renal cysts: comparative evaluation with MR imaging. Radiology. 1987;162(3):679–84. doi: https://doi.org/10.1148/radiology.162.3.3809481.
Nishiura JL, Eloi SR, Heilberg IP. Pain determinants of pain in autosomal dominant polycystic kidney disease. J Bras Nefrol. 2013;35(3):242–3. doi: https://doi.org/10.5935/0101-2800.20130038.
Ozkok A, Akpinar TS, Tufan F, Kanitez NA, Uysal M, Guzel M, et al. Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: a single center experience. Clin Exp Nephrol. 2013;17(3):345–51. doi: https://doi.org/10.1007/s10157-012-0706-3.
Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287. doi: https://doi.org/10.1136/bmj.e5287.
Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016;13(11):654–62. doi: https://doi.org/10.1038/nrurol.2016.154.
Fishman MC, Pollack HM, Arger PH, Banner MP. High protein content: another cause of CT hyperdense benign renal cyst. J Comput Assist Tomogr. 1983;7(6):1103–6.
. Health Effects of Exposure to Low Levels of Ionizing Radiations: Time for Reassessment? Washington (DC)1998.
Wang Y, Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med. 2015;73(1):82–101. doi: https://doi.org/10.1002/mrm.25358.
Harada T, Kudo K, Fujima N, Yoshikawa M, Ikebe Y, Sato R, et al. Quantitative Susceptibility Mapping: Basic Methods and Clinical Applications. Radiographics. 2022;42(4):1161–76. doi: https://doi.org/10.1148/rg.210054.
Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012;59(3):2560–8. doi: https://doi.org/10.1016/j.neuroimage.2011.08.082.
de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, et al. Quantitative susceptibility map reconstruction from MR phase data using Bayesian regularization: validation and application to brain imaging. Magn Reson Med. 2010;63(1):194–206. doi: https://doi.org/10.1002/mrm.22187.
Chen W, Zhu W, Kovanlikaya I, Kovanlikaya A, Liu T, Wang S, et al. Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping. Radiology. 2014;270(2):496–505. doi: https://doi.org/10.1148/radiol.13122640.
Dimov AV, Li J, Nguyen TD, Roberts AG, Spincemaille P, Straub S, et al. QSM Throughout the Body. J Magn Reson Imaging. 2023;57(6):1621–40. doi: https://doi.org/10.1002/jmri.28624.
Eskreis-Winkler S, Zhang Y, Zhang J, Liu Z, Dimov A, Gupta A, Wang Y. The clinical utility of QSM: disease diagnosis, medical management, and surgical planning. NMR Biomed. 2017;30(4). doi: https://doi.org/10.1002/nbm.3668.
Fuller S, Reeder S, Shimakawa A, Yu H, Johnson J, Beaulieu C, Gold GE. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fast spin-echo imaging of the ankle: initial clinical experience. AJR Am J Roentgenol. 2006;187(6):1442–7. doi: https://doi.org/10.2214/AJR.05.0930.
Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, Pelc NJ. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med. 2004;51(1):35–45. doi: https://doi.org/10.1002/mrm.10675.
Dong J, Liu T, Chen F, Zhou D, Dimov A, Raj A, et al. Simultaneous phase unwrapping and removal of chemical shift (SPURS) using graph cuts: application in quantitative susceptibility mapping. IEEE Trans Med Imaging. 2015;34(2):531–40. doi: https://doi.org/10.1109/TMI.2014.2361764.
Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205–12. doi: https://doi.org/10.1681/ASN.2008050507.
Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343(8901):824–7. doi: https://doi.org/10.1016/s0140-6736(94)92026-5.
Eskreis-Winkler S, Corrias G, Monti S, Zheng J, Capanu M, Krebs S, et al. IDEAL-IQ in an oncologic population: meeting the challenge of concomitant liver fat and liver iron. Cancer Imaging. 2018;18(1):51. doi: https://doi.org/10.1186/s40644-018-0167-3.
Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med. 2013;69(2):467–76. doi: https://doi.org/10.1002/mrm.24272.
Liu T, Khalidov I, de Rochefort L, Spincemaille P, Liu J, Tsiouris AJ, Wang Y. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011;24(9):1129–36. doi: https://doi.org/10.1002/nbm.1670.
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. doi: https://doi.org/10.1016/j.neuroimage.2006.01.015.
Roubidoux MA. MR imaging of hemorrhage and iron deposition in the kidney. Radiographics. 1994;14(5):1033–44. doi: https://doi.org/10.1148/radiographics.14.5.7991812.
Vinayagamani S, Sheelakumari R, Sabarish S, Senthilvelan S, Ros R, Thomas B, Kesavadas C. Quantitative Susceptibility Mapping: Technical Considerations and Clinical Applications in Neuroimaging. J Magn Reson Imaging. 2021;53(1):23–37. doi: https://doi.org/10.1002/jmri.27058.
Ravanfar P, Loi SM, Syeda WT, Van Rheenen TE, Bush AI, Desmond P, et al. Systematic Review: Quantitative Susceptibility Mapping (QSM) of Brain Iron Profile in Neurodegenerative Diseases. Front Neurosci. 2021;15:618435. doi: https://doi.org/10.3389/fnins.2021.618435.
Liu C, Wei H, Gong NJ, Cronin M, Dibb R, Decker K. Quantitative Susceptibility Mapping: Contrast Mechanisms and Clinical Applications. Tomography. 2015;1(1):3–17. doi: https://doi.org/10.18383/j.tom.2015.00136.
Nikparast F, Ganji Z, Zare H. Early differentiation of neurodegenerative diseases using the novel QSM technique: what is the biomarker of each disorder? BMC Neurosci. 2022;23(1):48. doi: https://doi.org/10.1186/s12868-022-00725-9.
Bandt SK, de Rochefort L, Chen WW, Dimov AV, Spincemaille P, Kopell BH, et al. Clinical Integration of Quantitative Susceptibility Mapping Magnetic Resonance Imaging into Neurosurgical Practice. World Neurosurg. 2019;122:E10–E9. doi: https://doi.org/10.1016/j.wneu.2018.08.213.
Agnello F, Albano D, Micci G, Di Buono G, Agrusa A, Salvaggio G, et al. CT and MR imaging of cystic renal lesions. Insights Imaging. 2020;11(1):5. doi: https://doi.org/10.1186/s13244-019-0826-3.
Balci NC, Semelka RC, Patt RH, Dubois D, Freeman JA, Gomez-Caminero A, Woosley JT. Complex renal cysts: findings on MR imaging. AJR Am J Roentgenol. 1999;172(6):1495–500. doi: https://doi.org/10.2214/ajr.172.6.10350279.
Bae KT, Grantham JJ. Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2010;6(2):96–106. doi: https://doi.org/10.1038/nrneph.2009.214.
Sallee M, Rafat C, Zahar JR, Paulmier B, Grunfeld JP, Knebelmann B, Fakhouri F. Cyst infections in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4(7):1183–9. doi: https://doi.org/10.2215/CJN.01870309.
Gibson P, Watson ML. Cyst infection in polycystic kidney disease: a clinical challenge. Nephrol Dial Transplant. 1998;13(10):2455–7. doi: https://doi.org/10.1093/ndt/13.10.2455.
Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, et al. Calibration of myocardial T2 and T1 against iron concentration. J Cardiovasc Magn Reson. 2014;16(1):62. doi: https://doi.org/10.1186/s12968-014-0062-4.
Kamman RL, Go KG, Brouwer W, Berendsen HJ. Nuclear magnetic resonance relaxation in experimental brain edema: effects of water concentration, protein concentration, and temperature. Magn Reson Med. 1988;6(3):265–74. doi: https://doi.org/10.1002/mrm.1910060304.
Jensen JH, Chandra R. Strong field behavior of the NMR signal from magnetically heterogeneous tissues. Magn Reson Med. 2000;43(2):226–36. doi: https://doi.org/10.1002/(sici)1522-2594(200002)43:2<226::aid-mrm9>3.0.co;2-p.
Luz Z, Meiboom S. Nuclear Magnetic Resonance Study of Protolysis of Trimethylammonium Ion in Aqueous Solution - Order of Reaction with Respect to Solvent. J Chem Phys. 1963;39(2):366-. Doi https://doi.org/10.1063/1.1734254.
Frahm J, Haase A, Matthaei D. Rapid NMR imaging of dynamic processes using the FLASH technique. Magn Reson Med. 1986;3(2):321–7. doi: https://doi.org/10.1002/mrm.1910030217.
Bernstein MA, King KF, Zhou ZJ. Handbook of MRI pulse sequences. Amsterdam; Boston: Academic Press; 2004.
Shi H, Jia J, Li D, Wei L, Shang W, Zheng Z. Blood oxygen level-dependent magnetic resonance imaging for detecting pathological patterns in patients with lupus nephritis: a preliminary study using gray-level co-occurrence matrix analysis. J Int Med Res. 2018;46(1):204–18. doi: https://doi.org/10.1177/0300060517721794.
Mie MB, Nissen JC, Zollner FG, Heilmann M, Schoenberg SO, Michaely HJ, Schad LR. Susceptibility weighted imaging (SWI) of the kidney at 3T--initial results. Z Med Phys. 2010;20(2):143–50. doi: https://doi.org/10.1016/j.zemedi.2010.02.004.
Pan L, Chen J, Xing W, Xing Z, Zhang J, Peng Y, Zhang Z. Magnetic resonance imaging evaluation of renal ischaemia–reperfusion injury in a rabbit model. Exp Physiol. 2017;102(8):1000–6. doi: https://doi.org/10.1113/EP086203.
Zhang JG, Xing ZY, Zha TT, Tian XJ, Du YN, Chen J, Xing W. Longitudinal assessment of rabbit renal fibrosis induced by unilateral ureteral obstruction using two-dimensional susceptibility weighted imaging. J Magn Reson Imaging. 2018;47(6):1572–7. doi: https://doi.org/10.1002/jmri.25915.
Li J, Chang S, Liu T, Wang Q, Cui D, Chen X, et al. Reducing the object orientation dependence of susceptibility effects in gradient echo MRI through quantitative susceptibility mapping. Magn Reson Med. 2012;68(5):1563–9. doi: https://doi.org/10.1002/mrm.24135.
Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med. 1994;32(6):749–63. doi: https://doi.org/10.1002/mrm.1910320610.
Dimov AV, Li J, Nguyen TD, Roberts AG, Spincemaille P, Straub S, et al. QSM Throughout the Body. J Magn Reson Imaging. 2023. doi: https://doi.org/10.1002/jmri.28624.
White RM. Quantum theory of magnetism: magnetic properties of materials. 3rd, completely rev. ed. Springer series in solid-state sciences. Berlin; New York: Springer; 2007.
Pauling L. General chemistry. New York: Dover Publications, Inc.; 1988.
Kazancioglu R, Ecder T, Altintepe L, Altiparmak MR, Tuglular S, Uyanik A, et al. Demographic and clinical characteristics of patients with autosomal dominant polycystic kidney disease: a multicenter experience. Nephron Clin Pract. 2011;117(3):c270–5. doi: https://doi.org/10.1159/000320745.
Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(4):838–44. doi: https://doi.org/10.2215/CJN.03100608.
Torres VE, Wilson DM, Hattery RR, Segura JW. Renal stone disease in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1993;22(4):513–9. doi: https://doi.org/10.1016/s0272-6386(12)80922-x.
Yaman S, Tekin HC. Magnetic Susceptibility-Based Protein Detection Using Magnetic Levitation. Anal Chem. 2020;92(18):12556–63. doi: https://doi.org/10.1021/acs.analchem.0c02479.
Chang S, Zhang J, Liu T, Tsiouris AJ, Shou J, Nguyen T, et al. Quantitative Susceptibility Mapping of Intracerebral Hemorrhages at Various Stages. J Magn Reson Imaging. 2016;44(2):420–5. doi: https://doi.org/10.1002/jmri.25143.
Bradley WG, Jr. MR appearance of hemorrhage in the brain. Radiology. 1993;189(1):15–26. doi: https://doi.org/10.1148/radiology.189.1.8372185.
Langkammer C, Liu T, Khalil M, Enzinger C, Jehna M, Fuchs S, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267(2):551–9. doi: https://doi.org/10.1148/radiol.12120707.
Choi Y, Jang J, Kim J, Nam Y, Shin NY, Ahn KJ, et al. MRI and Quantitative Magnetic Susceptibility Maps of the Brain after Serial Administration of Gadobutrol: A Longitudinal Follow-up Study. Radiology. 2020;297(1):143–50. doi: https://doi.org/10.1148/radiol.2020192579.
Liu S, Wang C, Zhang X, Zuo P, Hu J, Haacke EM, Ni H. Quantification of liver iron concentration using the apparent susceptibility of hepatic vessels. Quant Imaging Med Surg. 2018;8(2):123–34. doi: https://doi.org/10.21037/qims.2018.03.02.
Wei H, Dibb R, Zhou Y, Sun Y, Xu J, Wang N, Liu C. Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed. 2015;28(10):1294–303. doi: https://doi.org/10.1002/nbm.3383.
Liu Z, Kee Y, Zhou D, Wang Y, Spincemaille P. Preconditioned total field inversion (TFI) method for quantitative susceptibility mapping. Magn Reson Med. 2017;78(1):303–15. doi: https://doi.org/10.1002/mrm.26331.
Kim W, Shin HG, Lee H, Park D, Kang J, Nam Y, et al. Chi-Separation Imaging for Diagnosis of Multiple Sclerosis versus Neuromyelitis Optica Spectrum Disorder. Radiology. 2023;307(1):e220941. doi: https://doi.org/10.1148/radiol.220941.
Chen J, Gong NJ, Chaim KT, Otaduy MCG, Liu C. Decompose quantitative susceptibility mapping (QSM) to sub-voxel diamagnetic and paramagnetic components based on gradient-echo MRI data. Neuroimage. 2021;242:118477. doi: https://doi.org/10.1016/j.neuroimage.2021.118477.
Dimov AV, Nguyen TD, Gillen KM, Marcille M, Spincemaille P, Pitt D, et al. Susceptibility source separation from gradient echo data using magnitude decay modeling. J Neuroimaging. 2022;32(5):852–9. doi: https://doi.org/10.1111/jon.13014.
Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160–72. doi: https://doi.org/10.1681/ASN.2013101138.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schumacher, K., Prince, M.R., Blumenfeld, J.D. et al. Quantitative susceptibility mapping for detection of kidney stones, hemorrhage differentiation, and cyst classification in ADPKD. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04243-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04243-6