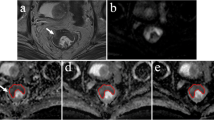
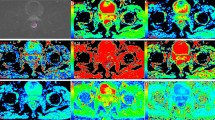
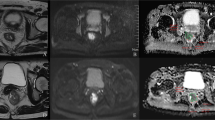
Abstract
Purpose
This study explored models of monoexponential diffusion-weighted imaging (DWI), diffusion kurtosis imaging (DKI), stretched exponential (SEM), fractional-order calculus (FROC), and continuous-time random-walk (CTRW) as diagnostic tools for assessing pathological prognostic factors in patients with resectable rectal cancer (RRC).
Methods
RRC patients who underwent radical surgery were included. The apparent diffusion coefficient (ADC), the mean kurtosis (MK) and mean diffusion (MD) from the DKI model, the distributed diffusion coefficient (DDC) and α from the SEM model, D, β and u from the FROC model, and D, α and β from the CTRW model were assessed.
Results
There were a total of 181 patients. The area under the receiver operating characteristic (ROC) curve (AUC) of CTRW-α for predicting histology type was significantly higher than that of FROC-u (0.780 vs. 0.671, p = 0.043). The AUC of CTRW-α for predicting pT stage was significantly higher than that of FROC-u and ADC (0.786 vs.0.683, p = 0.043; 0.786 vs. 0.682, p = 0.030), the difference in predictive efficacy of FROC-u between ADC and MK was not statistically significant [0.683 vs. 0.682, p = 0.981; 0.683 vs. 0.703, p = 0.720]; the difference between the predictive efficacy of MK and ADC was not statistically significant (p = 0.696). The AUC of CTRW (α + β) (0.781) was significantly higher than that of FROC-u (0.781 vs. 0.625, p = 0.003) in predicting pN stage but not significantly different from that of MK (p = 0.108).
Conclusion
The CTRW and DKI models may serve as imaging biomarkers to predict pathological prognostic factors in RRC patients before surgery.
Graphical abstract




Similar content being viewed by others
References
Asgeirsson T, Zhang S, Senagore AJ (2010) Optimal follow-up to curative colon and rectal cancer surgery: how and for how long? Surgical oncology clinics of North America 19:861-873 https://doi.org/10.1016/j.soc.2010.06.003
Lee YC, Hsieh CC, Chuang JP (2013) Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum 56:1093-1101 https://doi.org/10.1097/DCR.0b013e318298e36b
Boras Z, Kondza G, Sisljagić V, Busić Z, Gmajnić R, Istvanić T (2012) Prognostic factors of local recurrence and survival after curative rectal cancer surgery: a single institution experience. Collegium antropologicum 36:1355-1361
Madbouly KM, Abbas KS, Hussein AM (2014) Metastatic lymph node ratio in stage III rectal carcinoma is a valuable prognostic factor even with less than 12 lymph nodes retrieved: a prospective study. American journal of surgery 207:824-831 https://doi.org/10.1016/j.amjsurg.2013.07.022
Sun RJ, Wang L, Li XT et al (2020) Baseline MRI detected lateral lymph node as a prognostic factor: a cohort study in pN0 low-risk rectal cancer. Journal of cancer research and clinical oncology 146:739-748 https://doi.org/10.1007/s00432-019-03100-0
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR. American journal of roentgenology 188:1622-1635 https://doi.org/10.2214/ajr.06.1403
Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG (2012) Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 35:1365-1371 https://doi.org/10.1002/jmri.23589
Akashi M, Nakahusa Y, Yakabe T et al (2014) Assessment of aggressiveness of rectal cancer using 3-T MRI: correlation between the apparent diffusion coefficient as a potential imaging biomarker and histologic prognostic factors. Acta radiologica (Stockholm, Sweden : 1987) 55:524–531 https://doi.org/10.1177/0284185113503154
Sun Y, Tong T, Cai S, Bi R, Xin C, Gu Y (2014) Apparent Diffusion Coefficient (ADC) value: a potential imaging biomarker that reflects the biological features of rectal cancer. PLoS One 9:e109371 https://doi.org/10.1371/journal.pone.0109371
Tang L, Zhou XJ (2019) Diffusion MRI of cancer: From low to high b-values. J Magn Reson Imaging 49:23-40 https://doi.org/10.1002/jmri.26293
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR in biomedicine 23:698-710 https://doi.org/10.1002/nbm.1518
Magin RL, Hall MG, Karaman MM, Vegh V (2020) Fractional Calculus Models of Magnetic Resonance Phenomena: Relaxation and Diffusion. Crit Rev Biomed Eng 48:285-326 https://doi.org/10.1615/CritRevBiomedEng.2020033925
Chen X, Jiang J, Shen N et al (2018) Stretched-exponential model diffusion-weighted imaging as a potential imaging marker in preoperative grading and assessment of proliferative activity of gliomas. Am J Transl Res 10:2659-2668
Zhang J, Weaver TE, Zhong Z et al (2019) White matter structural differences in OSA patients experiencing residual daytime sleepiness with high CPAP use: a non-Gaussian diffusion MRI study. Sleep medicine 53:51-59 https://doi.org/10.1016/j.sleep.2018.09.011
Gao A, Zhang H, Yan X et al (2022) Whole-Tumor Histogram Analysis of Multiple Diffusion Metrics for Glioma Genotyping. Radiology 302:E16 https://doi.org/10.1148/radiol.219034
Guo H, Liu J, Hu J et al (2022) Diagnostic performance of gliomas grading and IDH status decoding A comparison between 3D amide proton transfer APT and four diffusion-weighted MRI models. J Magn Reson Imaging 56:1834-1844 https://doi.org/10.1002/jmri.28211
Huang Z, Li X, Wang Z et al (2022) Application of Simultaneous (18) F-FDG PET With Monoexponential, Biexponential, and Stretched Exponential Model-Based Diffusion-Weighted MR Imaging in Assessing the Proliferation Status of Lung Adenocarcinoma. J Magn Reson Imaging 56:63-74 https://doi.org/10.1002/jmri.28010
Wang C, Wang G, Zhang Y et al (2023) Differentiation of benign and malignant breast lesions using diffusion-weighted imaging with a fractional-order calculus model. European journal of radiology 159:110646 https://doi.org/10.1016/j.ejrad.2022.110646
Qin Y, Tang C, Hu Q, Yi J, Yin T, Ai T (2023) Assessment of Prognostic Factors and Molecular Subtypes of Breast Cancer With a Continuous-Time Random-Walk MR Diffusion Model: Using Whole Tumor Histogram Analysis. J Magn Reson Imaging 58:93-105 https://doi.org/10.1002/jmri.28474
Seo N, Chung YE, Park YN, Kim E, Hwang J, Kim MJ (2018) Liver fibrosis: stretched exponential model outperforms mono-exponential and bi-exponential models of diffusion-weighted MRI. Eur Radiol 28:2812-2822 https://doi.org/10.1007/s00330-017-5292-z
Liu G, Lu Y, Dai Y et al (2021) Comparison of mono-exponential, bi-exponential, kurtosis, and fractional-order calculus models of diffusion-weighted imaging in characterizing prostate lesions in transition zone. Abdom Radiol (NY) 46:2740-2750 https://doi.org/10.1007/s00261-020-02903-x
Feng C, Wang Y, Dan G et al (2022) Evaluation of a fractional-order calculus diffusion model and bi-parametric VI-RADS for staging and grading bladder urothelial carcinoma. Eur Radiol 32:890-900 https://doi.org/10.1007/s00330-021-08203-2
Wang P, Weng L, Xie S et al (2021) Primary application of mean apparent propagator-MRI diffusion model in the grading of diffuse glioma. European journal of radiology 138:109622 https://doi.org/10.1016/j.ejrad.2021.109622
Daducci A, Canales-Rodríguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran JP (2015) Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. NeuroImage 105:32-44 https://doi.org/10.1016/j.neuroimage.2014.10.026
Lian S, Liu H, Meng T, Ma L, Zeng W, Xie C (2023) Quantitative synthetic MRI for predicting locally advanced rectal cancer response to neoadjuvant chemoradiotherapy. Eur Radiol 33:1737-1745 https://doi.org/10.1007/s00330-022-09191-7
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology 17:1471–1474 https://doi.org/10.1245/s10434-010-0985-4
Nagtegaal ID, Odze RD, Klimstra D et al (2020) The 2019 WHO classification of tumours of the digestive system. Histopathology 76:182-188 https://doi.org/10.1111/his.13975
Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G (2013) The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the "DISTANCE"? Radiology 268:330-344 https://doi.org/10.1148/radiol.13121361
Peng Y, Li Z, Tang H et al (2018) Comparison of reduced field-of-view diffusion-weighted imaging (DWI) and conventional DWI techniques in the assessment of rectal carcinoma at 3.0T: Image quality and histological T staging. J Magn Reson Imaging 47:967-975 https://doi.org/10.1002/jmri.25814
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32-35 https://doi.org/10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837-845
Hu S, Peng Y, Wang Q et al (2022) T2*-weighted imaging and diffusion kurtosis imaging (DKI) of rectal cancer: correlation with clinical histopathologic prognostic factors. Abdom Radiol (NY) 47:517-529 https://doi.org/10.1007/s00261-021-03369-1
Li H, Chen GW, Liu YS et al (2020) Assessment of histologic prognostic factors of resectable rectal cancer: comparison of diagnostic performance using various apparent diffusion coefficient parameters. Sci Rep 10:11554 https://doi.org/10.1038/s41598-020-68328-0
Heijnen LA, Lambregts DM, Mondal D et al (2013) Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol 23:3354-3360 https://doi.org/10.1007/s00330-013-2952-5
Le Bihan D (2013) Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 268:318-322 https://doi.org/10.1148/radiol.13130420
Huang Y, Lin Y, Hu W et al (2019) Diffusion Kurtosis at 3.0T as an in vivo Imaging Marker for Breast Cancer Characterization: Correlation With Prognostic Factors. J Magn Reson Imaging 49:845-856 https://doi.org/10.1002/jmri.26249
Yuan J, Gong Z, Liu K et al (2022) Correlation between diffusion kurtosis and intravoxel incoherent motion derived (IVIM) parameters and tumor tissue composition in rectal cancer: a pilot study. Abdom Radiol (NY) 47:1223-1231 https://doi.org/10.1007/s00261-022-03426-3
Zhu L, Pan Z, Ma Q et al (2017) Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology 284:66-76 https://doi.org/10.1148/radiol.2016160094
Yang L, Xia C, Zhao J, Zhou X, Wu B (2021) The value of intravoxel incoherent motion and diffusion kurtosis imaging in the assessment of tumor regression grade and T stages after neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. European journal of radiology 136:109504 https://doi.org/10.1016/j.ejrad.2020.109504
Zhang XY, Wang L, Zhu HT et al (2020) Predicting Rectal Cancer Response to Neoadjuvant Chemoradiotherapy Using Deep Learning of Diffusion Kurtosis MRI. Radiology 296:56-64 https://doi.org/10.1148/radiol.2020190936
Karaman MM, Sui Y, Wang H, Magin RL, Li Y, Zhou XJ (2016) Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn Reson Med 76:1149-1157 https://doi.org/10.1002/mrm.26012
Qin Y, Tang C, Hu Q, Yi J, Yin T, Ai T (2022) Assessment of Prognostic Factors and Molecular Subtypes of Breast Cancer With a Continuous-Time Random-Walk MR Diffusion Model: Using Whole Tumor Histogram Analysis. J Magn Reson Imaging https://doi.org/10.1002/jmri.28474
Zhou M, Pu H, Chen MN, Wang YT (2023) Feasibility of Simultaneous Multislice Acceleration Technique in Readout-Segmented Echo-Planar Diffusion-Weighted Imaging for Assessing Rectal Cancer. Diagnostics (Basel) 13: https://doi.org/10.3390/diagnostics13030474
Funding
No funding was received for the conduct of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, M., Bao, D., Huang, H. et al. Utilization of diffusion-weighted derived mathematical models to predict prognostic factors of resectable rectal cancer. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04239-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04239-2