Abstract
Background
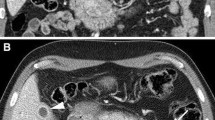
Primary sclerosing cholangitis (PSC) is a cholestatic liver disease that progresses to cirrhosis and liver failure. The Anali and Amsterdam scores are based upon imaging features on MRI and ERCP, respectively.
Aims
We aimed to compare the interobserver variability and performances of these scores.
Methods
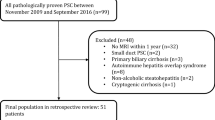
Patients with PSC with at least 1 MRCP were included. Images were independently scored by 2 experts. Agreement and prognostic performance with a primary end point of hepatic decompensation was assessed.
Results
Fifty-nine patients were included (67.8% male, 86.4% IBD). Interobserver agreement for the Anali and Amsterdam scores were moderate (k = 0.49; 95% CI 0.35–0.64 and k = 0.43; 95% CI 0.30–0.56, respectively). Among the Anali components, dysmorphy (caudate/right lobe ratio > 0.9) had fair agreement (k = 0.37; 95% CI 0.14–0.60) and portal hypertension (k = 0.64, 95% CI 0.32–0.89) and intrahepatic dilation (k = 0.70; 95% CI 0.53–0.87) had substantial agreement. The Amsterdam extrahepatic and intrahepatic scores had fair agreement (k = 0.38; 95% CI 0.23–0.52) and moderate agreement (k = 0.50; 95% CI 0.34–0.67), respectively. Anali score (HR 5.90, 95% CI 1.64–21.21), total bilirubin (HR = 3.23; 95% Cl 1.06–9.91), and age (HR = 1.05; 95% CI 1.00–1.11) were independent predictors of hepatic decompensation. Mayo risk score and Anali score had good discriminative ability with c-statistics of 0.78 (CI 0.59–0.96) and 0.76 (CI 0.56–0.91). Anali score remained an independent predictor after adjusting for Mayo risk score.
Conclusion
Anali score adds additional predictive value for hepatic decompensation in patients with PSC.
Graphical abstract




Similar content being viewed by others
References
Bowlus, C.L., et al., AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology, 2022.
Trivedi, P.J., et al., Epidemiology, Natural History, and Outcomes of Primary Sclerosing Cholangitis: A Systematic Review of Population-based Studies. Clin Gastroenterol Hepatol, 2022. 20(8): p. 1687–1700 e4.
Ponsioen, C.Y., et al., Defining Primary Sclerosing Cholangitis: Results From an International Primary Sclerosing Cholangitis Study Group Consensus Process. Gastroenterology, 2021. 161(6): p. 1764-1775.
Kim, W.R., et al., A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc, 2000. 75(7): p. 688-94.
Eaton, J.E., et al., Primary Sclerosing Cholangitis Risk Estimate Tool (PREsTo) Predicts Outcomes of the Disease: A Derivation and Validation Study Using Machine Learning. Hepatology, 2020. 71(1): p. 214-224.
de Vries, E.M., et al., A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut, 2018. 67(10): p. 1864-1869.
de Vries, E.M., et al., Validation of the prognostic value of histologic scoring systems in primary sclerosing cholangitis: An international cohort study. Hepatology, 2017. 65(3): p. 907-919.
Corpechot, C., et al., Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology, 2014. 146(4): p. 970-9; quiz e15-6.
Ehlken, H., et al., Validation of Transient Elastography and Comparison with Spleen Length Measurement for Staging of Fibrosis and Clinical Prognosis in Primary Sclerosing Cholangitis. PLoS One, 2016. 11(10): p. e0164224.
Eaton, J.E., et al., Changes in Liver Stiffness, Measured by Magnetic Resonance Elastography, Associated With Hepatic Decompensation in Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol, 2020. 18(7): p. 1576-1583 e1.
Ruiz, A., et al., Radiologic course of primary sclerosing cholangitis: assessment by three-dimensional magnetic resonance cholangiography and predictive features of progression. Hepatology, 2014. 59(1): p. 242-50.
Cazzagon, N., et al., Quantitative magnetic resonance cholangiopancreatography metrics are associated with disease severity and outcomes in people with primary sclerosing cholangitis. JHEP Rep, 2022. 4(11): p. 100577.
Cristoferi, L., et al., A quantitative MRCP-derived score for medium-term outcome prediction in primary sclerosing cholangitis. Dig Liver Dis, 2022.
Ismail, M.F., et al., Evaluation of quantitative MRCP (MRCP+) for risk stratification of primary sclerosing cholangitis: comparison with morphological MRCP, MR elastography, and biochemical risk scores. Eur Radiol, 2022. 32(1): p. 67-77.
Ponsioen, C.Y., et al., Validation of a cholangiographic prognostic model in primary sclerosing cholangitis. Endoscopy, 2010. 42(9): p. 742-7.
Tenca, A., et al., The role of magnetic resonance imaging and endoscopic retrograde cholangiography in the evaluation of disease activity and severity in primary sclerosing cholangitis. Liver Int, 2018. 38(12): p. 2329-2339.
Lemoinne, S., et al., Simple Magnetic Resonance Scores Associate With Outcomes of Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol, 2019. 17(13): p. 2785-2792.e3.
Grigoriadis, A., et al., Assessment of prognostic value and interreader agreement of ANALI scores in patients with primary sclerosing cholangitis. Eur J Radiol, 2021. 142: p. 109884.
Grigoriadis, A., et al., Inter-reader agreement of interpretation of radiological course of bile duct changes between serial follow-up magnetic resonance imaging/3D magnetic resonance cholangiopancreatography of patients with primary sclerosing cholangitis. Scand J Gastroenterol, 2020. 55(2): p. 228-235.
Selvaraj, E.A., et al., A Quantitative Magnetic Resonance Cholangiopancreatography Metric of Intrahepatic Biliary Dilatation Severity Detects High-Risk Primary Sclerosing Cholangitis. Hepatol Commun, 2022. 6(4): p. 795-808.
Awaya, H., et al., Cirrhosis: modified caudate-right lobe ratio. Radiology, 2002. 224(3): p. 769-74.
Zenouzi, R., et al., Follow-up magnetic resonance imaging/3D-magnetic resonance cholangiopancreatography in patients with primary sclerosing cholangitis: challenging for experts to interpret. Aliment Pharmacol Ther, 2018. 48(2): p. 169-178.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CLB has received grants from Boston Scientific, Calliditas Therapeutics, Cara Therapeutics, ChemoMab, COUR Pharmaceuticals, Cymabay Therapeutics, Gilead Sciences, GSK Pharmaceuticals, Hanmi Pharmaceuticals, Intercept Pharmaceuticals, Ipsen Bioscience, Mirum Pharmaceuticals, Novo Nordisk, Pliant Therapeutics, and Viking Therapeutics. CLB has been an advisor for Cymabay Therapeutics, GSK Pharmaceuticals, Invea Therapeutics, and Ipsen Bioscience. MTC is a consultant for Corcept Therapeutics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grave, E.C., Loehfelm, T., Corwin, M.T. et al. Interobserver agreement and prognostic value of image-based scoring systems in patients with primary sclerosing cholangitis. Abdom Radiol 49, 60–68 (2024). https://doi.org/10.1007/s00261-023-04051-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04051-4