Abstract
Purpose
To investigate the image quality and diagnostic performance of low-contrast-dose liver CT using a deep learning-based iodine contrast-augmenting algorithm (DLICA) for hypovascular hepatic metastases.
Methods
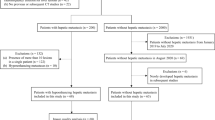
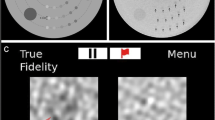
This retrospective study included 128 patients who underwent contrast-enhanced dual-energy CT for hepatic metastasis surveillance between July 2019 and June 2022 using a 30% reduced iodine contrast dose in the portal phase. Three image types were reconstructed: 50-keV virtual monoenergetic images (50-keV VMI); linearly blended images simulating 120-kVp images (120-kVp); and post-processed 120-kVp images using DLICA (DLICA 120-kVp). Three reviewers evaluated lesion conspicuity, image contrast, and subjective image noise. We also measured image noise, contrast-to-noise ratios (CNRs), and signal-to-noise ratios (SNRs). The diagnostic performance for hepatic metastases was evaluated using a jackknife alternative free-response receiver operating characteristic method with the consensus of two independent radiologists as the reference standard.
Results
DLICA 120-kVp demonstrated significantly higher CNR of lesions to liver (5.7 ± 3.1 vs. 3.8 ± 2.1 vs. 3.8 ± 2.1) and higher SNR compared with 50-keV VMI and 120-kVp (p < 0.001 for all). DLICA 120-kVp had significantly lower image noise than 50-kVp VMI for all regions (p < 0.001 for all). DLICA 120-kVp also exhibited superior lesion conspicuity (4.0 [3.3–4.3] vs. 3.7 [3.0–4.0] vs. 3.7 [3.0–4.0]), higher image contrast, and lower subjective image noise compared with 50-keV VMI and 120-kVp (p < 0.001 for all). Although there was no significant difference in the figure of merit for lesion diagnosis among the three methods (p = 0.11), DLICA 120-kVp had a significantly higher figure of merit for lesions with a diameter < 20 mm than 50-keV VMI (0.677 vs. 0.648, p = 0.007). On a per-lesion basis, DLICA 120-kVp also demonstrated higher sensitivity than the 50-keV VMI (81.2% vs. 72.9%, p < 0.001). The specificities per lesion were not significantly different among the three algorithms (p = 0.15).
Conclusion
DLICA at 120-kVp provided superior lesion conspicuity and image quality and similar diagnostic performance for hypovascular hepatic metastases compared with 50-keV VMI.
Graphical abstract






Similar content being viewed by others
References
Schima W, Kulinna C, Langenberger H, Ba-Ssalamah A (2005) Liver metastases of colorectal cancer: US, CT or MR? Cancer Imaging 5 Spec No A:S149–S156. https://doi.org/10.1102/1470-7330.2005.0035
Haubold J, Hosch R, Umutlu L et al (2021) Contrast agent dose reduction in computed tomography with deep learning using a conditional generative adversarial network. Eur Radiol 31:6087–6095. https://doi.org/10.1007/s00330-021-07714-2
Hanson GJ, Michalak GJ, Childs R et al (2018) Low kV versus dual-energy virtual monoenergetic CT imaging for proven liver lesions: what are the advantages and trade-offs in conspicuity and image quality? A pilot study. Abdom Radiol (NY) 43:1404–1412. https://doi.org/10.1007/s00261-017-1327-9
De Cecco CN, Darnell A, Rengo M et al (2012) Dual-energy CT: oncologic applications. AJR Am J Roentgenol 199:S98–S105. https://doi.org/10.2214/ajr.12.9207
Albrecht MH, Vogl TJ, Martin SS et al (2019) Review of clinical applications for virtual monoenergetic dual-energy CT. Radiology 293:260–271. https://doi.org/10.1148/radiol.2019182297
Nagayama Y, Nakaura T, Oda S et al (2018) Dual-layer DECT for multiphasic hepatic CT with 50 percent iodine load: a matched-pair comparison with a 120 kVp protocol. Eur Radiol 28:1719–1730. https://doi.org/10.1007/s00330-017-5114-3
Lenga L, Czwikla R, Wichmann JL et al (2018) Dual-energy CT in patients with colorectal cancer: improved assessment of hypoattenuating liver metastases using noise-optimized virtual monoenergetic imaging. Eur J Radiol 106:184-191. https://doi.org/10.1016/j.ejrad.2018.07.027
Lenga L, Lange M, Arendt CT et al (2021) Can dual-energy CT-based virtual monoenergetic imaging improve the assessment of hypodense liver metastases in patients with hepatic steatosis? Acad Radiol 28:769–777. https://doi.org/10.1016/j.acra.2020.03.044
Martin SS, Pfeifer S, Wichmann JL et al (2017) Noise-optimized virtual monoenergetic dual-energy computed tomography: optimization of kiloelectron volt settings in patients with gastrointestinal stromal tumors. Abdom Radiol (NY) 42:718–726. https://doi.org/10.1007/s00261-016-1011-5
Fink MA, Seibold C, Kauczor HU, Stiefelhagen R, Kleesiek J (2022) Jointly optimized deep neural networks to synthesize monoenergetic images from single-energy CT angiography for improving classification of pulmonary embolism. Diagnostics (Basel) 12. https://doi.org/10.3390/diagnostics12051224
Gong H, Marsh JF, D'Souza KN et al (2021) Deep-learning-based direct synthesis of low-energy virtual monoenergetic images with multi-energy CT. J Med Imaging (Bellingham) 8:052104. https://doi.org/10.1117/1.jmi.8.5.052104
Kang HJ, Lee JM, Ahn C et al (2023) Low dose of contrast agent and low radiation liver computed tomography with deep-learning-based contrast boosting model in participants at high-risk for hepatocellular carcinoma: prospective, randomized, double-blind study. Eur Radiol 33:3660–3670. https://doi.org/10.1007/s00330-023-09520-4
Sica GT (2006) Bias in research studies. Radiology 238:780–789. https://doi.org/10.1148/radiol.2383041109
Caruso D, De Cecco CN, Schoepf UJ et al (2017) Can dual-energy computed tomography improve visualization of hypoenhancing liver lesions in portal venous phase? Assessment of advanced image-based virtual monoenergetic images. Clin Imaging 41:118–124. https://doi.org/10.1016/j.clinimag.2016.10.015
Tao S, Rajendran K, Zhou W, Fletcher JG, McCollough CH, Leng S (2019) Improving iodine contrast to noise ratio using virtual monoenergetic imaging and prior-knowledge-aware iterative denoising (mono-PKAID). Phys Med Biol 64:105014. https://doi.org/10.1088/1361-6560/ab17fa
Krauss B, Grant KL, Schmidt BT, Flohr TG (2015) The importance of spectral separation: an assessment of dual-energy spectral separation for quantitative ability and dose efficiency. Invest Radiol 50:114–118. https://doi.org/10.1097/rli.0000000000000109
Lee T, Lee JM, Yoon JH et al (2022) Deep learning-based image reconstruction of 40-keV virtual monoenergetic images of dual-energy CT for the assessment of hypoenhancing hepatic metastasis. Eur Radiol 32:6407–6417. https://doi.org/10.1007/s00330-022-08728-0
Park S, Yoon JH, Joo I et al (2022) Image quality in liver CT: low-dose deep learning vs standard-dose model-based iterative reconstructions. Eur Radiol 32:2865–2874. https://doi.org/10.1007/s00330-021-08380-0
Borjesson S, Hakansson M, Bath M et al (2005) A software tool for increased efficiency in observer performance studies in radiology. Radiat Prot Dosimetry 114:45–52. https://doi.org/10.1093/rpd/nch550
Jensen CT, Gupta S, Saleh MM et al (2022) Reduced-dose deep learning reconstruction for abdominal CT of liver metastases. Radiology 303:90–98. https://doi.org/10.1148/radiol.211838
Robinson E, Babb J, Chandarana H, Macari M (2010) Dual source dual energy MDCT: comparison of 80 kVp and weighted average 120 kVp data for conspicuity of hypo-vascular liver metastases. Invest Radiol 45:413–418. https://doi.org/10.1097/rli.0b013e3181dfda78
Yamada Y, Jinzaki M, Tanami Y, Abe T, Kuribayashi S (2012) Virtual monochromatic spectral imaging for the evaluation of hypovascular hepatic metastases: the optimal monochromatic level with fast kilovoltage switching dual-energy computed tomography. Invest Radiol 47:292–298. https://doi.org/10.1097/rli.0b013e318240a874
Park J, Shin J, Min IK et al (2022) Image quality and lesion detectability of lower-dose abdominopelvic CT obtained using deep learning image reconstruction. Korean J Radiol 23(4):402–412. https://doi.org/10.3348/kjr.2021.0683
Chen FM, Ni JM, Zhang ZY, Zhang L, Li B, Jiang CJ (2016) Presurgical evaluation of pancreatic cancer: a comprehensive imaging comparison of CT versus MRI. AJR Am J Roentgenol 206:526–535. https://doi.org/10.2214/ajr.15.15236
Funding
This study was supported by a research grant from ClariPi (No. 06-2022-4530).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Chulkyun Ahn is an employee of ClariPi. His role was confined to providing the DLICA image sets, and he did not participate in data analysis. Other authors declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
This retrospective study was approved by the Institutional Review Board of Seoul National University Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, T., Yoon, J.H., Park, J.Y. et al. Deep learning-based iodine contrast-augmenting algorithm for low-contrast-dose liver CT to assess hypovascular hepatic metastasis. Abdom Radiol 48, 3430–3440 (2023). https://doi.org/10.1007/s00261-023-04039-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04039-0