Abstract
Purpose
To compare MiraLAX, a hypo-osmotic lavage, and magnesium citrate (MgC), a hyper-osmotic agent for bowel preparation at CTC.
Methods
398 total screening CTC studies were included in this retrospective, single institution study. 297 underwent preparation with a double-dose MgC regimen (mean age, 61 ± 5.5 years; 142 male/155 female) and 101 with 8.3 oz (equivalent to 238 g PEG) of MiraLAX (mean age, 60 ± 9.6 years; 45 male/56 female). Oral contrast for tagging purposes was utilized in both regimens. Studies were retrospectively analyzed for residual fluid volume and attenuation by automated analysis, as well for subjective oral contrast coating of the normal colonic wall and polyps. 50 patients underwent successive CTC studies utilizing each agent (mean, 6.1 ± 1.7 years apart), allowing for intra-patient comparison. Chi-squared, Fisher’s exact, McNemar, and t-tests were used for data comparison.
Results
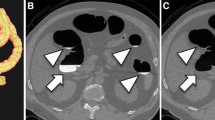
Residual fluid volume (as percentage of total colonic volume) and fluid density was 7.2 ± 4.2% and 713 ± 183 HU for the MgC cohort and 8.7 ± 3.8% and 1044 HU ± 274 for the MiraLAX cohort, respectively (p = 0.001 and p < 0.001, respectively). Similar results were observed for the intra-patient cohort. Colonic wall coating negatively influencing interpretation was noted in 1.7% of MgC vs. 6.9% of MiraLAX examinations (p = 0.008). Polyps were detected in 12% of all MgC vs. 16% of all MiraLAX CTCs (p = 0.29).
Conclusion
CTC bowel preparation with the hypo-osmotic MiraLAX agent appears to provide acceptable diagnostic quality that is comparable to the hyper-osmotic MgC agent, especially when factoring in patient safety and tolerance.





Similar content being viewed by others
References
Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007; 357:1403-1412
de Haan MC, Pickhardt PJ, Stoker J. CT colonography: accuracy, acceptance, safety and position in organised population screening. Gut 2015; 64:342-350
Pickhardt PJ. Imaging and Screening for Colorectal Cancer with CT Colonography. Radiol Clin North Am 2017; 55:1183-1196
Ricci ZJ, Kobi M, Flusberg M, Yee J. CT Colonography in Review With Tips and Tricks to Improve Performance. Semin Roentgenol 2021; 56:140-151
Spada C, Hassan C, Bellini D, et al. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline - Update 2020. Eur Radiol 2021; 31:2967-2982
Pickhardt PJ. CT colonography for population screening: ready for prime time? Dig Dis Sci 2015; 60:647-659
Johnson B, Hinshaw JL, Robbins JB, Pickhardt PJ. Objective and Subjective Intrapatient Comparison of Iohexol Versus Diatrizoate for Bowel Preparation Quality at CT Colonography. AJR Am J Roentgenol 2016; 206:1202-1207
Bannas P, Bakke J, Munoz del Rio A, Pickhardt PJ. Intra-individual comparison of magnesium citrate and sodium phosphate for bowel preparation at CT colonography: automated volumetric analysis of residual fluid for quality assessment. Clin Radiol 2014; 69:1171-1177
Bannas P, Bakke J, Patrick JL, Pickhardt PJ. Automated volumetric analysis for comparison of oral sulfate solution (SUPREP) with established cathartic agents at CT colonography. Abdom Imaging 2015; 40:11-18
Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel preparation for CT colonography: blinded comparison of magnesium citrate and sodium phosphate for catharsis. Radiology 2010; 254:138-144
Pickhardt PJ, Choi JH. Electronic cleansing and stool tagging in CT colonography: advantages and pitfalls with primary three-dimensional evaluation. AJR Am J Roentgenol 2003; 181:799-805
Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT colonography. AJR Am J Roentgenol 2007; 189:1451-1456
Kim DH, Moreno CC, Pickhardt PJ. Computed Tomography Colonography: Pearls and Pitfalls. Radiol Clin North Am 2018; 56:719-735
Pickhardt PJ, Kim DH. CT colonography: pitfalls in interpretation. Radiol Clin North Am 2013; 51:69-88
Slater A, Taylor SA, Burling D, Gartner L, Scarth J, Halligan S. Colonic polyps: effect of attenuation of tagged fluid and viewing window on conspicuity and measurement--in vitro experiment with porcine colonic specimen. Radiology 2006; 240:101-109
Kim DH, Lubner MG, Cahoon AR, Pooler BD, Pickhardt PJ. Flat Serrated Polyps at CT Colonography: Relevance, Appearance, and Optimizing Interpretation. Radiographics 2018; 38:60-74
Kim DH, Matkowskyj KA, Lubner MG, et al. Serrated Polyps at CT Colonography: Prevalence and Characteristics of the Serrated Polyp Spectrum. Radiology 2016; 280:455-463
Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy 2019; 51:775-794
LLC V-J. Vi-Jon, LLC Expands Voluntary Nationwide Recall of Magnesium Citrate Saline Laxative Oral Solution Lemon Flavor Due to Microbial Contamination. In: FDA, 2022
Burling D, Taylor SA, Halligan S, et al. Automated insufflation of carbon dioxide for MDCT colonography: distension and patient experience compared with manual insufflation. AJR Am J Roentgenol 2006; 186:96-103
Michel SJ, Pickhardt PJ, Kim DH, Taylor AJ. Effect of colonic distention on superiority of supine versus prone views in screening computed tomographic colonography. Clin Imaging 2007; 31:325-328
Jung SH. Stratified Fisher's exact test and its sample size calculation. Biom J 2014; 56:129-140
Vi-Jon, LLC Expands Voluntary Nationwide Recall of Magnesium Citrate Saline Laxative Oral Solution Lemon Flavor Due to Microbial Contamination. In: FDA, 2022
Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003; 349:2191-2200
Reumkens A, van der Zander Q, Winkens B, et al. Electrolyte disturbances after bowel preparation for colonoscopy: Systematic review and meta-analysis. Dig Endosc 2022; 34:913-926
Saltzman JR, Cash BD, Pasha SF, et al. Bowel preparation before colonoscopy. Gastrointest Endosc 2015; 81:781-794
Nyberg C, Hendel J, Nielsen OH. The safety of osmotically acting cathartics in colonic cleansing. Nat Rev Gastroenterol Hepatol 2010; 7:557-564
Keedy AW, Yee J, Aslam R, et al. Reduced cathartic bowel preparation for CT colonography: prospective comparison of 2-L polyethylene glycol and magnesium citrate. Radiology 2011; 261:156-164
Jin Z, Lu Y, Zhou Y, Gong B. Systematic review and meta-analysis: sodium picosulfate/magnesium citrate vs. polyethylene glycol for colonoscopy preparation. Eur J Clin Pharmacol 2016; 72:523-532
Matro R, Daskalakis C, Negoianu D, et al. Randomised clinical trial: Polyethylene glycol 3350 with sports drink vs. polyethylene glycol with electrolyte solution as purgatives for colonoscopy--the incidence of hyponatraemia. Aliment Pharmacol Ther 2014; 40:610-619
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
P.J.P serves as an advisor to Bracco Diagnostics, Nanox-AI, and GE Healthcare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zacharias, N., Lubner, M.G., Kim, D.H. et al. Comparison of MiraLAX and magnesium citrate for bowel preparation at CT colonography. Abdom Radiol 48, 3322–3331 (2023). https://doi.org/10.1007/s00261-023-04025-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04025-6