Abstract
Purpose
To assess the ability of an automated AI tool to detect intravenous contrast material (IVCM) in abdominal CT examinations using spleen attenuation.
Methods
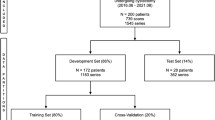
A previously validated automated AI tool measuring the attenuation of the spleen was deployed on a sample of 32,994 adult (age ≥ 18) patients (mean age, 61.9 ± 14.7 years; 13,869 men, 19,125 women) undergoing 65,449 supine position CT examinations (41,020 with and 24,429 without IVCM by DICOM header) from January 1, 2000 to December 31, 2021. After exclusions, receiver operating characteristic (ROC) curve analysis was performed to determine the optimal threshold for binary classification of IVCM status (non-contrast vs IVCM enhanced), which was then applied to the sample. Discordant examinations (i.e., IVCM status determined by AI tool did not match DICOM header) were manually reviewed to establish ground truth. Repeat ROC curve and contingency table analysis were performed to assess AI tool performance.
Results
ROC analysis of the initial study sample of 61,783 CT examinations yielded AUC of 0.970 with Youden index suggesting an optimal spleen attenuation threshold of 65 Hounsfield units (HU). Manual review of 2094 discordant CT examinations revealed discordance due to DICOM header error in 1278 (61.0%) and AI tool misclassification in 410 (19.6%), with 406 (9.4%) meeting exclusion criteria. Analysis of 61,377 CT examinations in the final study sample yielded AUC of 0.999 with accuracy of 99.3% at the 65 HU threshold. Error rate for DICOM header information was 2.1% (1278/61,377) versus 0.7% (410/61,377) for the AI tool.
Conclusion
The automated spleen attenuation AI tool was highly accurate for detection of IVCM at a threshold of 65 HU.






Similar content being viewed by others
References
Deo RC. Machine Learning in Medicine. Circulation 2015; 132:1920–1930
Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med 2019; 25:44-56
Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H. Artificial intelligence in radiology. Nature Reviews Cancer 2018; 18:500-510
Pickhardt PJ, Graffy PM, Perez AA, Lubner MG, Elton DC, Summers RM. Opportunistic Screening at Abdominal CT: Use of Automated Body Composition Biomarkers for Added Cardiometabolic Value. Radiographics 2021; 41:524-542
Pickhardt PJ, Graffy PM, Zea R, et al. Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: a retrospective cohort study. Lancet Digit Health 2020; 2:e192-e200
Pickhardt PJ, Graffy PM, Zea R, et al. Automated Abdominal CT Imaging Biomarkers for Opportunistic Prediction of Future Major Osteoporotic Fractures in Asymptomatic Adults. Radiology 2020; 297:64-72
Pickhardt PJ, Graffy PM, Zea R, et al. Utilizing Fully Automated Abdominal CT-Based Biomarkers for Opportunistic Screening for Metabolic Syndrome in Adults Without Symptoms. AJR Am J Roentgenol 2021; 216:85-92
Magudia K, Bridge CP, Andriole KP, Rosenthal MH. The Trials and Tribulations of Assembling Large Medical Imaging Datasets for Machine Learning Applications. J Digit Imaging 2021; 34:1424-1429
Perez AA, Pickhardt PJ, Elton DC, Sandfort V, Summers RM. Fully automated CT imaging biomarkers of bone, muscle, and fat: correcting for the effect of intravenous contrast. Abdominal radiology (New York) 2021; 46:1229-1235
Robertson F, Leander P, Ekberg O. Radiology of the spleen. European Radiology 2001; 11:80-95
Elsayes KM, Narra VR, Mukundan G, Lewis JS, Menias CO, Heiken JP. MR imaging of the spleen: Spectrum of abnormalities. Radiographics 2005; 25:967-982
Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. In: Ourselin S, Joskowicz L, Sabuncu MR, Unal G, Wells W, eds. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2016. Cham: Springer International Publishing, 2016:424-432
Kayalıbay B, Jensen G, van der Smagt P. CNN-based Segmentation of Medical Imaging Data. 2017
Lee S, Elton DC, Yang AH, et al. Fully Automated and Explainable Liver Segmental Volume Ratio and Spleen Segmentation at CT for Diagnosing Cirrhosis. Radiol Artif Intell 2022; 4:e210268
Sandfort V, Yan K, Pickhardt P, Summers R. Data augmentation using generative adversarial networks (CycleGAN) to improve generalizability in CT segmentation tasks. Scientific Reports 2019; 9
Lubner MG, Graffy PM, Said A, et al. Utility of Multiparametric CT for Identification of High-Risk NAFLD. AJR Am J Roentgenol 2021; 216:659-668
Yan K, Lu L, Summers RM. Unsupervised body part regression via spatially self-ordering convolutional neural networks. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018) 2018:1022-1025
Yan K, Wang X, Lu L, et al. Deep Lesion Graphs in the Wild: Relationship Learning and Organization of Significant Radiology Image Findings in a Diverse Large-scale Lesion Database. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition 2018:9261-9270
Hanley JA, McNeil BJ. The Meaning and Use of the Area Under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982; 143:29-36
Hanley JA, McNeil BJ. Method of Comparing the Areas Under Receiver Operating Characteristic Curves Derived from the Same Cases. Radiology 1983; 148:839-843
Smith AD. Automated Screening for Future Osteoporotic Fractures on Abdominal CT: Opportunistic or an Outstanding Opportunity? Radiology 2020; 297:73-74
Ye Z, Qian JM, Hosny A, et al. Deep Learning-based Detection of Intravenous Contrast Enhancement on CT Scans. Radiol Artif Intell 2022; 4:e210285
Dietvorst BJ, Simmons JP, Massey C. Algorithm aversion: people erroneously avoid algorithms after seeing them err. J Exp Psychol Gen 2015; 144:114-126
Pooler BD, Garrett JW, Southard AM, Summers RM, Pickhardt PJ. Technical Adequacy of Fully Automated Artificial Intelligence Body Composition Tools: Assessment in a Heterogeneous Sample of External CT Examinations. AJR Am J Roentgenol 2023:1-9
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health Clinical Center.
Author information
Authors and Affiliations
Contributions
All authors (BDP, CJF, JWG, RMS, PJP) participated in and contributed materially to the design of this study, as well as manuscript writing, production, and editing. The decision to publish and final submitted manuscript was approved by all authors. Data collection was conducted by JWG, CJF, and BDP. Data and statistical analysis were conducted principally by BDP and JWG. BDP and PJP serve as joint guarantors for the study and accept full responsibility for the finished work.
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests or conflicts of interest for any author. The authors wish to disclose the following non-competing interests: BDP, CJF, and JWG: nothing to disclose; PJP: advisor to Bracco, GE Healthcare, and Nano-X. RMS receives royalties from iCAD, ScanMed, PingAn, Philips, and Translation Holdings. His lab received research funding through a Cooperative Research and Development Agreement with PingAn.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pooler, B.D., Fleming, C.J., Garrett, J.W. et al. Artificial intelligence tool detection of intravenous contrast enhancement using spleen attenuation. Abdom Radiol 48, 3382–3390 (2023). https://doi.org/10.1007/s00261-023-04020-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04020-x