Abstract
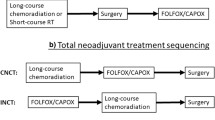
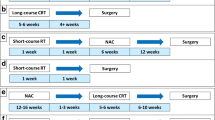
Total neoadjuvant therapy (TNT), which includes chemotherapy and radiation prior to surgical resection, has been recently accepted as the new standard of care for patients with locally advanced low and mid rectal cancers. Multiple clinical trials have evaluated this approach in the last several decades and demonstrated improvement in, local control and reduced risk of recurrence. In addition, in the course of these investigations, it has been shown that between a third and a half of patients experience a clinical complete response (cCR) after being treated with the TNT approach, leading to the development of new organ preservation protocol, now known as watch-and-wait (W&W). On this protocol, cCR patients are not referred for surgery after total neoadjuvant treatment. Instead, they remain on close surveillance and, thus, avoid potential complications associated with surgical resection. Multiple clinical trials are ongoing, investigating the long-term outcomes of these new approaches and the development of less toxic and more effective TNT regimens for LARC. Improvements in technology and rectal MRI protocols position radiologists as vital members of multidisciplinary rectal cancer management teams. Rectal MRI has become a critical tool for rectal cancer initial staging, treatment response assessment, and surveillance on W&W protocols. In this review, we summarize the findings of the pivotal clinical trials that contributed to establishing the current treatment paradigms in locally advanced rectal cancer (LARC) management, with the intention of helping radiologists play more effective roles in their multidisciplinary teams.
Similar content being viewed by others
References
Little, R.G., 2nd, L.A. Ebertowski, and C.S. David, Inhibition of alloantigen presentation by cyclosporine. Transplantation, 1990. 49(5): p. 937–44.
Peeters, K.C., et al., Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol, 2005. 23(25): p. 6199-206.
Birgisson, H., et al., Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol, 2005. 23(25): p. 6126-31.
Glynne-Jones, R., et al., Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2017. 28(suppl_4): p. iv22-iv40.
Taylor, F.G., et al., Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg, 2011. 253(4): p. 711-9.
Ruppert, R., et al., Avoidance of Overtreatment of Rectal Cancer by Selective Chemoradiotherapy: Results of the Optimized Surgery and MRI-Based Multimodal Therapy Trial. J Am Coll Surg, 2020. 231(4): p. 413-425.e2.
Shahida Ahmed, N.B., Alexandre Bouchard, James Brierley, Carl Brown, Gina Brown, Selliah Kanthan, Zane Cohen, Bernard Cummings, Ray Deobald, Sébastien Drolet, Stan Feinberg, Darlene Fenech, Dan Gill, David Hochman, Kartik Jhaveri, Erin Kennedy, Richard Kirsch, Neil Kopek, Vijayananda Kundapur, Eric Leung, Sender Liberman, Tony MacLean, Victoria Marcus, Alex Mathieson, Robin McLeod, Stanislas Morin, Catherine O'Brien, Michael Ott, Nikhilesh Patil, Anat Ravid, Marko Simunovic, Peter Stotland, Seng Thipphavong, Lara Williams, QuickSilver: A Phase II Study Using Magnetic Resonance Imaging Criteria to Identify "Good Prognosis" Rectal Cancer Patients Eligible for Primary Surgery. JMIR Res Protoc, 2015. 4(2): p. e41.
Kaur, H., et al., MRI Staging in an Evolving Management Paradigm for Rectal Cancer, From the AJR Special Series on Cancer Staging. American Journal of Roentgenology, 2021. 217(6): p. 1282-1293.
Brouwer, N.P.M., et al., Clinical lymph node staging in colorectal cancer; a flip of the coin? European Journal of Surgical Oncology, 2018. 44(8): p. 1241-1246.
Stelzner, S., et al., Selection of patients with rectal cancer for neoadjuvant therapy using pre-therapeutic MRI – Results from OCUM trial. European Journal of Radiology, 2022. 147.
The Ocum Group, Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM Rectal Cancer Trial. British Journal of Surgery, 2018. 105(11): p. 1519-1529.
Kennedy, E.D., et al., Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients With "Good Prognosis" Rectal Cancer Eligible for Primary Surgery: The Phase 2 Nonrandomized QuickSilver Clinical Trial. JAMA Oncol, 2019. 5(7): p. 961-966.
Sauer, R., et al., Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med, 2004. 351(17): p. 1731-40.
Watanabe, T., et al., Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol, 2018. 23(1): p. 1-34.
Fujita, S., et al., Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol, 2012. 13(6): p. 616-21.
Fujita, S., et al., Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg, 2017. 266(2): p. 201-207.
Tsukamoto, S., et al., Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg, 2020. 107(5): p. 586-594.
Kusters, M., et al., A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg, 2009. 249(2): p. 229-35.
Kim, T.H., et al., Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol, 2008. 15(3): p. 729-37.
Ogura, A., et al., Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol, 2019. 37(1): p. 33-43.
Ohue, M., et al., A Phase II/III randomized controlled trial comparing perioperative versus postoperative chemotherapy with mFOLFOX6 for lower rectal cancer with suspected lateral pelvic node metastasis: Japan Clinical Oncology Group Study JCOG1310 (PRECIOUS study). Jpn J Clin Oncol, 2017. 47(1): p. 84-87.
Conroy, T., et al., Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 2021. 22(5): p. 702-715.
Bahadoer, R.R., et al., Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol, 2021. 22(1): p. 29-42.
Jin, J., et al., Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol, 2022. 40(15): p. 1681-1692.
Garcia-Aguilar, J., et al., Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol, 2022. 40(23): p. 2546-2556.
Goffredo, P., et al., Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers (Basel), 2022. 14(13).
Fokas, E., et al., Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol, 2019. 37(34): p. 3212-3222.
Fokas, E., et al., Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol, 2022. 8(1): p. e215445.
Cercek, A., et al., Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol, 2018. 4(6): p. e180071.
Rullier, E., et al., Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol, 2020. 5(5): p. 465-474.
Rouanet, P., et al., Tailored Strategy for Locally Advanced Rectal Carcinoma (GRECCAR 4): Long-term Results From a Multicenter, Randomized, Open-Label, Phase II Trial. Dis Colon Rectum, 2022. 65(8): p. 986-995.
Brouquet, A., et al., NORAD01-GRECCAR16 multicenter phase III non-inferiority randomized trial comparing preoperative modified FOLFIRINOX without irradiation to radiochemotherapy for resectable locally advanced rectal cancer (intergroup FRENCH-GRECCAR- PRODIGE trial). BMC Cancer, 2020. 20(1): p. 485.
Habr-Gama, A., et al., Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg, 2004. 240(4): p. 711–7; discussion 717–8.
"Watch and Wait" After Neo-adjuvant Chemoradiotherapy for Primary Locally Advanced Rectal Cancer. (NORWAIT). 2020 January 7, 2023]; Available from: https://www.clinicaltrials.gov/ct2/show/NCT03402477?term=NORWAIT&draw=2&rank=1.
van der Valk, M.J.M., et al., Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet, 2018. 391(10139): p. 2537-2545.
Smith, J.J., et al., Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol, 2019. 5(4): p. e185896.
Kennecke, H.F., et al., Neoadjuvant Chemotherapy, Excision, and Observation for Early Rectal Cancer: The Phase II NEO Trial (CCTG CO.28) Primary End Point Results. Journal of Clinical Oncology, 2022. 41(2): p. 233–242.
WoW Watch and wait: Clinical complete response after (chemo)radiotherapy in advanced rectal cancer: A multicentre prospective national cohort study. 2017; Available from: http://www.ssorg.net/files/6815/7062/6019/Studieprotokoll_WoW_190207.pdf.
Bahadoer, R.R., et al., Interpreting the RAPIDO trial: factors to consider - Authors' reply. Lancet Oncol, 2021. 22(3): p. e90-e91.
Karoui, M., et al., Perioperative FOLFOX 4 Versus FOLFOX 4 Plus Cetuximab Versus Immediate Surgery for High-Risk Stage II and III Colon Cancers: A Phase II Multicenter Randomized Controlled Trial (PRODIGE 22). Ann Surg, 2020. 271(4): p. 637-645.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paroder, V., Fraum, T.J., Nougaret, S. et al. Key clinical trials in rectal cancer shaping the current treatment paradigms: reference guide for radiologists. Abdom Radiol 48, 2825–2835 (2023). https://doi.org/10.1007/s00261-023-03931-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03931-z