Abstract
Purpose
To externally validate an algorithm for non-invasive differentiation of hepatic mucinous cystic neoplasms (MCN) from benign hepatic cysts (BHC), which differ in management.
Methods
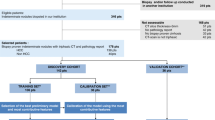
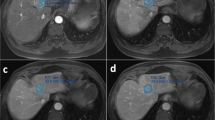
Patients with cystic liver lesions pathologically confirmed as MCN or BHC between January 2005 and March 2022 from multiple institutions were retrospectively included. Five readers (2 radiologists, 3 non-radiologist physicians) independently reviewed contrast-enhanced CT or MRI examinations before tissue sampling and applied the 3-feature classification algorithm described by Hardie et al. to differentiate between MCN and BHC, which had a reported accuracy of 93.5%. The classification was then compared to the pathology results. Interreader agreement between readers across different levels of experience was evaluated with Fleiss’ Kappa.
Results
The final cohort included 159 patients, median age of 62 years (IQR [52.0, 70.0]), 66.7% female (106). Of all patients, 89.3% (142) had BHC, and the remaining 10.7% (17) had MCN on pathology. Agreement for class designation between the radiologists was almost perfect (Fleiss’ Kappa 0.840, p < 0.001). The algorithm had an accuracy of 98.1% (95% CI [94.6%, 99.6%]), a positive predictive value of 100.0% (95% CI [76.8%, 100.0%]), a negative predictive value of 97.9% (95% CI [94.1%, 99.6%]), and an area under the receiver operator characteristic curve (AUC) of 0.911 (95% CI [0.818, 1.000]).
Conclusion
The evaluated algorithm showed similarly high diagnostic accuracy in our external, multi-institutional validation cohort. This 3-feature algorithm is easily and rapidly applied and its features are reproducible among radiologists, showing promise as a clinical decision support tool.
Graphical Abstract





Similar content being viewed by others
Data availability
The final de-identified dataset and the code used for the analyses reported in this study are available from the corresponding author upon reasonable request.
References
Soni S, Pareek P, Narayan S, Varshney V (2021) Mucinous cystic neoplasm of the liver (MCN-L): a rare presentation and review of the literature. Med Pharm Rep 94:366–371. https://doi.org/10.15386/mpr-1543
Mavilia MG, Pakala T, Molina M, Wu GY (2018) Differentiating cystic liver lesions: A review of imaging modalities, diagnosis and management. J Clin Transl Hepatol 6:1–9. https://doi.org/10.14218/jcth.2017.00069
Marrero JA, Ahn J, Rajender Reddy K, Americal College of Gastroenterology (2014) ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 109:1328–47; quiz 1348. https://doi.org/10.1038/ajg.2014.213
Lantinga MA, Gevers TJG, Drenth JPH (2013) Evaluation of hepatic cystic lesions. World J Gastroenterol 19:3543–3554. https://doi.org/https://doi.org/10.3748/wjg.v19.i23.3543
Vachha B, Sun MRM, Siewert B, Eisenberg RL (2011) Cystic lesions of the liver. AJR Am J Roentgenol 196:W355-66. https://doi.org/https://doi.org/10.2214/AJR.10.5292
Hardie AD, Chamberlin JH, Boyum JH, Sharbidre KG, Petrocelli R, Flemming BP, Zahid M, Venkatesh SK, Mruthyunjayappa S, Hajdu CH, Kovacs MD (2022) Multi-center follow-up study to develop a classification system which differentiates mucinous cystic neoplasm of the liver and benign hepatic cyst using machine learning. Acad Radiol 29:1149–1156. https://doi.org/https://doi.org/10.1016/j.acra.2021.08.025
Dua MM, Gerry J, Salles A, Tran TB, Triadafilopoulos G, Visser BC (2016) Biliary cystadenoma: A suggested “cystamatic” approach? Dig Dis Sci 61:1835–1838. https://doi.org/https://doi.org/10.1007/s10620-015-3943-y
Xu H-X, Lu M-D, Liu L-N, Zhang Y-F, Guo L-H, Liu C, Wang S (2012) Imaging features of intrahepatic biliary cystadenoma and cystadenocarcinoma on B-mode and contrast-enhanced ultrasound. Ultraschall Med 33:E241–E249. https://doi.org/https://doi.org/10.1055/s-0031-1299276
Dong Y, Wang W-P, Mao F, Fan M, Ignee A, Serra C, Sparchez Z, Sporea I, Braden B, Dietrich CF (2017) Contrast enhanced ultrasound features of hepatic cystadenoma and hepatic cystadenocarcinoma. Scand J Gastroenterol 52:365–372. https://doi.org/https://doi.org/10.1080/00365521.2016.1259652
Park HJ, Kim SY, Kim HJ, Lee SS, Hong GS, Byun JH, Hong S-M, Lee M-G (2018) Intraductal papillary neoplasm of the bile duct: Clinical, imaging, and pathologic features. AJR Am J Roentgenol 211:67–75. https://doi.org/https://doi.org/10.2214/AJR.17.19261
Le DK, Agarwal A (2019) Intraductal papillary neoplasm of the bile duct. Proc (Bayl Univ Med Cent) 32:124–125. https://doi.org/https://doi.org/10.1080/08998280.2018.1520623
Bakoyiannis A, Delis S, Triantopoulou C, Dervenis C (2013) Rare cystic liver lesions: a diagnostic and managing challenge. World J Gastroenterol 19:7603–7619. https://doi.org/https://doi.org/10.3748/wjg.v19.i43.7603
Rodriguez RM, Barrio M, Parker ML, Saeed O, Sherman S, Ceppa EP (2019) Mucinous cystic neoplasms of the liver: presence of biliary communication. J Surg Case Rep 2019:rjz364. https://doi.org/10.1093/jscr/rjz364
Chi H, Hansen BE, Tang WY, Schouten JNL, Sprengers D, Taimr P, Janssen HLA, de Knegt RJ (2017) Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur J Gastroenterol Hepatol 29:36–41. https://doi.org/https://doi.org/10.1097/MEG.0000000000000731
Myers RP, Fong A, Shaheen AAM (2008) Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int 28:705–712. https://doi.org/https://doi.org/10.1111/j.1478-3231.2008.01691.x
Boyum JH, Atwell TD, Schmit GD, Poterucha JJ, Schleck CD, Harmsen WS, Kamath PS (2016) Incidence and risk factors for adverse events related to image-guided liver biopsy. Mayo Clin Proc 91:329–335. https://doi.org/https://doi.org/10.1016/j.mayocp.2015.11.015
Anderson MA, Bhati CS, Ganeshan D, Itani M (2021) Hepatobiliary mucinous cystic neoplasms and mimics. Abdom Radiol (NY). https://doi.org/https://doi.org/10.1007/s00261-021-03303-5
Kim HJ, Yu ES, Byun JH, Hong S-M, Kim KW, Lee JS, Kim SY (2014) CT differentiation of mucin-producing cystic neoplasms of the liver from solitary bile duct cysts. AJR Am J Roentgenol 202:83–91. https://doi.org/https://doi.org/10.2214/AJR.12.9170
Anderson MA, Dhami RS, Fadzen CM, Molina G, Taylor MS, Deshpande V, Qadan M, Catalano OA, Ferrone CR, Mojtahed A (2021) CT and MRI features differentiating mucinous cystic neoplasms of the liver from pathologically simple cysts. Clin Imaging 76:46–52. https://doi.org/https://doi.org/10.1016/j.clinimag.2021.01.036
Arnaoutakis DJ, Kim Y, Pulitano C, Zaydfudim V, Squires MH, Kooby D, Groeschl R, Alexandrescu S, Bauer TW, Bloomston M, Soares K, Marques H, Gamblin TC, Popescu I, Adams R, Nagorney D, Barroso E, Maithel SK, Crawford M, Sandroussi C, Marsh W, Pawlik TM (2015) Management of biliary cystic tumors: a multi-institutional analysis of a rare liver tumor. Ann Surg 261:361–367. https://doi.org/https://doi.org/10.1097/SLA.0000000000000543
Choi HK, Lee JK, Lee KH, Lee KT, Rhee JC, Kim KH, Jang K-T, Kim SH, Park Y (2010) Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings. J Clin Gastroenterol 44:289–293. https://doi.org/https://doi.org/10.1097/MCG.0b013e3181b5c789
Seo JK, Kim SH, Lee SH, Park JK, Woo SM, Jeong JB, Hwang J-H, Ryu JK, Kim J-W, Jeong S-H, Kim Y-T, Yoon YB, Lee KU, Kim SH, Kim MA (2010) Appropriate diagnosis of biliary cystic tumors: comparison with atypical hepatic simple cysts. Eur J Gastroenterol Hepatol 22:989–996. https://doi.org/https://doi.org/10.1097/MEG.0b013e328337c971
Kim JY, Kim SH, Eun HW, Lee MW, Lee JY, Han JK, Choi BI (2010) Differentiation between biliary cystic neoplasms and simple cysts of the liver: accuracy of CT. AJR Am J Roentgenol 195:1142–1148. https://doi.org/https://doi.org/10.2214/AJR.09.4026
Kovacs MD, Sheafor DH, Burchett PF, Picard MM, Hardie AD (2018) Differentiating biliary cystadenomas from benign hepatic cysts: Preliminary analysis of new predictive imaging features. Clin Imaging 49:44–47. https://doi.org/https://doi.org/10.1016/j.clinimag.2017.10.022
Boyum JH, Sheedy SP, Graham RP, Olson JT, Babcock AT, Bolan CW, Menias CO, Venkatesh SK (2021) Hepatic mucinous cystic neoplasm versus simple biliary cyst: Assessment of distinguishing imaging features using CT and MRI. AJR Am J Roentgenol 216:403–411. https://doi.org/https://doi.org/10.2214/AJR.20.22768
Bleeker SE, Moll HA, Steyerberg EW, Donders ART, Derksen-Lubsen G, Grobbee DE, Moons KGM (2003) External validation is necessary in prediction research: a clinical example. J Clin Epidemiol 56:826–832. https://doi.org/https://doi.org/10.1016/s0895-4356(03)00207-5
Austin PC, Steyerberg EW (2017) Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat Methods Med Res 26:796–808. https://doi.org/https://doi.org/10.1177/0962280214558972
Steyerberg EW, Harrell FE Jr (2016) Prediction models need appropriate internal, internal–external, and external validation. J Clin Epidemiol 69:245–247. https://doi.org/https://doi.org/10.1016/j.jclinepi.2015.04.005
Steyerberg EW, Harrell FE Jr, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF (2001) Internal validation of predictive models. J Clin Epidemiol 54:774–781. https://doi.org/https://doi.org/10.1016/s0895-4356(01)00341-9
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med 22:276–282
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12:77. https://doi.org/https://doi.org/10.1186/1471-2105-12-77
R Core Team (2022) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing
RStudio Team (2022) RStudio: Integrated Development Environment for R. RStudio, PBC
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. https://doi.org/https://doi.org/10.2307/2529310
Yan W, Huang L, Xia L, Gu S, Yan F, Wang Y, Tao Q (2020) MRI manufacturer shift and adaptation: Increasing the generalizability of deep learning segmentation for MR images acquired with different scanners. Radiol Artif Intell 2:e190195. https://doi.org/10.1148/ryai.2020190195
Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK (2018) Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: A cross-sectional study. PLoS Med 15:e1002683. https://doi.org/10.1371/journal.pmed.1002683
Shah S, Mishra R, Szczurowska A, Guziński M (2021) Non-invasive multi-channel deep learning convolutional neural networks for localization and classification of common hepatic lesions. Pol J Radiol 86:e440–e448. https://doi.org/https://doi.org/10.5114/pjr.2021.108257
Yin J, Qiu J-J, Qian W, Ji L, Yang D, Jiang J-W, Wang J-R, Lan L (2020) A radiomics signature to identify malignant and benign liver tumors on plain CT images. J Xray Sci Technol 28:683–694. https://doi.org/https://doi.org/10.3233/XST-200675
Halimu C, Kasem A, Newaz SHS (2019) Empirical comparison of area under ROC curve (AUC) and Mathew correlation coefficient (MCC) for evaluating machine learning algorithms on imbalanced datasets for binary classification. In: Proceedings of the 3rd International Conference on Machine Learning and Soft Computing - ICMLSC 2019. ACM Press, New York, New York, USA
Acknowledgements
The authors thank Shawn Murphy, Henry Chueh, and the Mass General Brigham Health Care Research Patient Data Registry group for facilitating the use of their database.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FSF, ÁBR, MH, and LM. The first draft of the manuscript was written by FSF, ÁBR, MAA, and OAC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no financial or proprietary interests in any material discussed in this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board.
Informed consent
Informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Furtado, F.S., Badenes-Romero, Á., Hesami, M. et al. External validation of a machine learning based algorithm to differentiate hepatic mucinous cystic neoplasms from benign hepatic cysts. Abdom Radiol 48, 2311–2320 (2023). https://doi.org/10.1007/s00261-023-03907-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03907-z