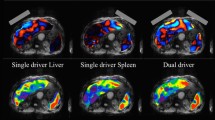
Abstract
Purpose
To evaluate the performance and repeatability assessing liver, spleen, and kidney stiffness with magnetic resonance elastography (MRE), using arrays of pneumatic passive drivers.
Methods
An array of four flexible, pneumatically activated passive drivers for abdominal MRE were developed and tested in this study. Multiple MRE acquisitions were performed prospectively in a series of eleven volunteers, with activation of all combinations of the four drivers, individually and simultaneously. MRE exams were repeated three times to study within-day and between-day test–retest repeatability. Semi-quantitative evaluation of wave propagation and penetration, and quantitative assessment of tissue stiffness was conducted for liver, spleen, and kidneys.
Results
When driver location and amplitude were sufficient to achieve necessary shear wave illumination in any given region of interest, the results showed excellent test–retest repeatability in abdominal organ stiffness with both single and multiple driver configurations. The results confirmed that multiple driver arrays provided suitable shear wave illumination over a larger region of the abdomen, allowing more reliable stiffness measurements in multiple organs. MRE assessment of the spleen was found to be prone to effects of excessive shear wave amplitude, however.
Conclusion
A multiple driver array provides shear wave illumination over a larger region of the abdomen than obtained with a single driver, for MRE assessment of multiple abdominal organs, providing excellent test–retest repeatability in stiffness measurements. However, careful tuning of the location and amplitude of each driver is essential to achieve consistent results.
Graphical abstract






Similar content being viewed by others
Abbreviations
- MRE:
-
Magnetic resonance elastography
- DR1:
-
Driver number 1
- DR2:
-
Driver number 2
- DR3:
-
Driver number 3
- DR4:
-
Driver number 4
- DR12:
-
Simultaneous activation of driver numbers 1 and 2
- DR13:
-
Simultaneous activation of driver numbers 1 and 3
- DR14:
-
Simultaneous activation of driver numbers 1 and 4
- DR1234:
-
Simultaneous activation of driver numbers 1, 2, 3, and 4
- IVPD:
-
Intravoxel phase dispersion
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- CV:
-
Coefficient of variation
- RC:
-
Repeatability coefficient
References
Hoodeshenas S, Yin M, Venkatesh SK. Magnetic Resonance Elastography of Liver: Current Update. Top Magn Reson Imaging. 2018;27:319–33.
Kennedy P, Stocker D, Carbonell G, Said D, Bane O, Hectors S, et al. MR elastography outperforms shear wave elastography for the diagnosis of clinically significant portal hypertension. Eur Radiol. 2022;32:8339–49.
Singh R, Wilson MP, Katlariwala P, Murad MH, McInnes MDF, Low G. Accuracy of liver and spleen stiffness on magnetic resonance elastography for detecting portal hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;32:237–45.
Morisaka H, Motosugi U, Ichikawa S, Sano K, Ichikawa T, Enomoto N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J Magn Reson Imaging. 2015;41:117–24.
Ronot M, Lambert S, Elkrief L, Doblas S, Rautou P-E, Castera L, et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24:1394–402.
Dyvorne HA, Jajamovich GH, Besa C, Cooper N, Taouli B. Simultaneous measurement of hepatic and splenic stiffness using MR elastography: preliminary experience. Abdom Imaging. 2015;40:803–9.
Bensamoun SF, Robert L, Leclerc GE, Debernard L, Charleux F. Stiffness imaging of the kidney and adjacent abdominal tissues measured simultaneously using magnetic resonance elastography. Clin Imaging. 2011;35:284–7.
Wang J, Deng Y, Jondal D, Woodrum DM, Shi Y, Yin M, et al. New and Emerging Applications of Magnetic Resonance Elastography of Other Abdominal Organs. Topics in Magnetic Resonance Imaging. 2018;27:335–52.
Zheng Y, Li G, Chen M, Chan QCC, Hu SG, Zhao XN, et al. Magnetic resonance elastography with twin pneumatic drivers for wave compensation. Annu Int Conf IEEE Eng Med Biol Soc. 2007;2007:2611–3.
Zheng Y, Chan QCC, Yang ES. Magnetic resonance elastography with twin drivers for high homogeneity and sensitivity. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:1916–9.
Mariappan YK, Rossman PJ, Glaser KJ, Manduca A, Ehman RL. Magnetic resonance elastography with a phased-array acoustic driver system. Magn Reson Med. 2009;61:678–85.
Neumann W, Lehnart VR, Vetter Y, Bichert A, Schad LR, Zöllner FG. Coupled actuators with a mechanically synchronized phase during MR elastography: A phantom feasibility study. Concepts Magn Reson Part B. 2018;48B:e21403.
Dittmann F, Tzschätzsch H, Hirsch S, Barnhill E, Braun J, Sack I, et al. Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states: Tomoelastography of the Abdomen. Magn Reson Med. 2017;78:976–83.
Silva AM, Grimm RC, Glaser KJ, Fu Y, Wu T, Ehman RL, et al. Magnetic resonance elastography: evaluation of new inversion algorithm and quantitative analysis method. Abdom Imaging. 2015;40:810–7.
Serai SD, Obuchowski NA, Venkatesh SK, Sirlin CB, Miller FH, Ashton E, et al. Repeatability of MR Elastography of Liver: A Meta-Analysis. Radiology. 2017;285:92–100.
Talwalkar JA, Yin M, Venkatesh S, Rossman PJ, Grimm RC, Manduca A, et al. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol. 2009;193:122–7.
Mannelli L, Godfrey E, Joubert I, Patterson AJ, Graves MJ, Gallagher FA, et al. MR elastography: Spleen stiffness measurements in healthy volunteers--preliminary experience. AJR Am J Roentgenol. 2010;195:387–92.
Gandhi D, Kalra P, Raterman B, Mo X, Dong H, Kolipaka A. Magnetic Resonance Elastography of kidneys: SE-EPI MRE reproducibility and its comparison to GRE MRE. NMR Biomed. 2019;32:e4141.
Acknowledgements
The authors thank Jennifer Kugel for assistance in preparing this manuscript.
Funding
This work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (grant numbers R37 EB001981, R01 EB017197), the Center for Individualized Medicine (CIM) at Mayo Clinic, and the Department of Defense (Grant No. W81XWH-19-1-0583-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jun Chen, Kevin J. Glaser, Roger Grimm, Richard L. Ehman, Meng Yin, and the Mayo Clinic have intellectual property rights and a financial interest related to magnetic resonance elastography technology.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of Mayo Clinic, and informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Chen, J., Heilman, J.A. et al. Abdominal MR elastography with multiple driver arrays: performance and repeatability. Abdom Radiol 48, 1945–1954 (2023). https://doi.org/10.1007/s00261-023-03866-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03866-5