Abstract
Objective
A log-combined model was developed to predict the invasive behavior of pancreatic solid pseudopapillary neoplasm (pSPN) based on clinical and radiomic features extracted from multiparametric magnetic resonance imaging (MRI).
Materials and methods
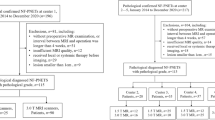
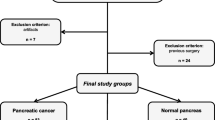
A total of 111 patients with pathologically confirmed pSPN who underwent preoperative plain and contrast-enhanced MRI were included, and divided into an invasive group (n = 34) and non-invasive group (n = 77). Clinical features and laboratory data related to pSPN invasive behavior were analyzed. Regions of interest were delineated based on T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and contrast-enhanced T1WI (CE-T1WI) to extract radiomic features. Correlation analysis was performed for these features, followed by L1_based feature selection (C = 0.15). A logistic regression algorithm was used to construct models based on each of the four sequences and a log-combined model was used to integrate the sequences. A receiver operating characteristic (ROC) curve was plotted to evaluate the model performance, and the Brier score was used to assess the overall accuracy of the model predictions.
Results
The area under the ROC curve was 0.68, 0.73, 0.71, and 0.49 for Log-T1WI, Log-T2WI, Log-DWI, and Log-CE models, respectively, and 0.81 for the log-combined model. The accuracy, precision, sensitivity, and specificity of the log-combined model were 0.77, 0.88, 0.75, and 0.78, respectively. The best performance was obtained with the log-combined model with a Brier score of 0.18. Tumor location was identified as a significant clinical feature in comparison between the two groups (p < 0.05), and invasive pSPN was more frequent in the tail of the pancreas.
Conclusion
The log-combined model based on multiparametric MRI and clinical features can be used as a non-invasive diagnostic tool for preoperative prediction of pSPN invasive behavior and to facilitate the development of individualized treatment strategies and monitoring management plans.
Graphical abstract





Similar content being viewed by others
References
[1] Guo N, Zhou QB, Chen RF, et al. Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: analysis of 24 ca ses. Can J Surg 2011; 54:368-374
[2] Yu P, Cheng X, Du Y, et al. Solid Pseudopapillary Neoplasms of the Pancreas: a 19-Year Multicenter Experience in China. J Gastrointest Surg 2015; 19:1433-1440
[3] Wang XG, Ni QF, Fei JG, Zhong ZX, Yu PF. Clinicopathologic features and surgical outcome of solid pseudopapillary tumor of the pancreas: analy sis of 17 cases. World J Surg Oncol 2013; 11:38
[4] Zhang C, Liu F, Chang H, et al. Less Aggressive Surgical Procedure for Treatment of Solid Pseudopapillary Tumor: Limited Experience f rom a Single Institute. PLoS One 2015; 10:e0143452
[5] Rathi J, Anuragi G, J RL, R P, C S, O LN. Prediction of Recurrence Risk in Solid Pseudopapillary Neoplasm of the Pancreas: Single-Institution Experience. Cureus 2021; 13:e17541
[6] Hosokawa I, Shimizu H, Ohtsuka M, et al. Preoperative diagnosis and surgical management for solid pseudopapillary neoplasm of the pancreas. J Hepatobiliary Pancreat Sci 2014; 21:573-578
[7] Yao J, Song H. A Review of Clinicopathological Characteristics and Treatment of Solid Pseudopapillary Tumor of the P ancreas with 2450 Cases in Chinese Population. Biomed Res Int 2020; 2020:2829647
[8] Kim MJ, Choi DW, Choi SH, Heo JS, Sung JY. Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br J Surg 2014; 101:1266-1271
[9] Sibio S, Di Carlo S. Current highlights on solid pseudopapillary neoplasm of the pancreas. World J Hepatol 2022; 14:300-303
[10] Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol 2018; 113:464-479
[11] Naar L, Spanomichou DA, Mastoraki A, Smyrniotis V, Arkadopoulos N. Solid Pseudopapillary Neoplasms of the Pancreas: A Surgical and Genetic Enigma. World J Surg 2017; 41:1871-1881
[12] Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with compon ents of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional c ases. Am J Surg Pathol 2005; 29:512-519
[13] Bhatti I, Ojo D, Dennison AR, Rees Y, Elabassy M, Garcea G. Percutaneous Pancreatic Biopsies-Still an Effective Method for Histologic Confirmation of Malignancy. Surg Laparosc Endosc Percutan Tech 2016; 26:334-337
[14] Hartwig W, Schneider L, Diener MK, Bergmann F, B¨1chler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg 2009; 96:5-20
[15] HooKim K, Reid MD. Atypical cells in fine needle aspiration biopsies of pancreas: Causes, work-up, and recommendations f or management. Diagn Cytopathol 2022; 50:196-207
[16] Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016; 278:563-577
[17] Compter I, Verduin M, Shi Z, et al. Deciphering the glioblastoma phenotype by computed tomography radiomics. Radiother Oncol 2021; 160:132-139
[18] Wang W, Cao K, Jin S, Zhu X, Ding J, Peng W. Differentiation of renal cell carcinoma subtypes through MRI-based radiomics analysis. Eur Radiol 2020; 30:5738-5747
[19] Li C, Yin J. Radiomics Nomogram Based on Radiomics Score from Multiregional Diffusion-Weighted MRI and Clinical Fa ctors for Evaluating HER-2 2+ Status of Breast Cancer. Diagnostics (Basel) 2021; 11
[20] Song T, Zhang QW, Duan SF, et al. MRI-based radiomics approach for differentiation of hypovascular non-functional pancreatic neuroendoc rine tumors and solid pseudopapillary neoplasms of the pancreas. BMC Med Imaging 2021; 21:36
[21] Benedetti G, Mori M, Panzeri MM, et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol Med 2021; 126:745-760
[22] Tobaly D, Santinha J, Sartoris R, et al. CT-Based Radiomics Analysis to Predict Malignancy in Patients with Intraductal Papillary Mucinous Neo plasm (IPMN) of the Pancreas. Cancers (Basel) 2020; 12
[23] Chakraborty J, Midya A, Gazit L, et al. CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med Phys 2018; 45:5019-5029
[24] Huang WP, Liu SY, Han YJ, Li LM, Liang P, Gao JB. Development of CT-Based Imaging Signature for Preoperative Prediction of Invasive Behavior in Pancrea tic Solid Pseudopapillary Neoplasm. Front Oncol 2021; 11:677814
[25] Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016; 15:155-163
[26] Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analy sis. J Clin Epidemiol 2001; 54:774-781
[27] Kang CM, Kim KS, Choi JS, Kim H, Lee WJ, Kim BR. Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas 2006; 32:276-280
[28] Wang J, Chen X, Wang C, et al. Differentiation of aggressive from non-aggressive pancreatic solid pseudopapillary neoplasms using co mputed tomography. Abdom Radiol (NY) 2019; 44:2448-2458
[29] Li Z, Pan D, Wang M, Ji Y, Zeng M. Application of MSCT characteristic nomogram model in predicting invasion of pancreatic solid pseudopa pillary neoplasms. Eur J Radiol 2022; 149:110201
[30] Rastogi A, Assing M, Taggart M, et al. Does Computed Tomography Have the Ability to Differentiate Aggressive From Nonaggressive Solid Pseudo papillary Neoplasm? J Comput Assist Tomogr 2018; 42:405-411
[31] Rastogi A, Assing M, Taggart M, et al. Does Computed Tomography Have the Ability to Differentiate Aggressive From Nonaggressive Solid Pseudopapillary Neoplasm? J Comput Assist Tomogr 2018; 42:405-411
[32] Raman SP, Kawamoto S, Law JK, et al. Institutional experience with solid pseudopapillary neoplasms: focus on computed tomography, magnetic resonance imaging, conventional ultrasound, endoscopic ultrasound, and predictors of aggressive his tology. J Comput Assist Tomogr 2013; 37:824-833
[33] Fusco R, Granata V, Mattace Raso M, et al. Blood Oxygenation Level Dependent Magnetic Resonance Imaging (MRI), Dynamic Contrast Enhanced MRI, an d Diffusion Weighted MRI for Benign and Malignant Breast Cancer Discrimination: A Preliminary Experi ence. Cancers (Basel) 2021; 13
[34] Lee JH, Yu JS, Kim H, et al. Solid pseudopapillary carcinoma of the pancreas: differentiation from benign solid pseudopapillary tumour using CT and MRI. Clin Radiol 2008; 63:1006-1014
[35] Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumor of pancreas: emphasis on magnetic resona nce imaging findings. World J Gastroenterol 2007; 13:1811-1815
[36] Ventriglia A, Manfredi R, Mehrabi S, et al. MRI features of solid pseudopapillary neoplasm of the pancreas. Abdom Imaging 2014; 39:1213-1220
[37] Abraham AS, Simon B, Eapen A, et al. Role of Cross-sectional Imaging (CT/MRI) in Characterization and Distinguishing Benign from Malignant /Potentially Malignant Cystic Lesions of Pancreas. J Clin Imaging Sci 2020; 10:28
[38] Shimizu T, Murata S, Mekata E, et al. Clinical potential of an antitumor drug sensitivity test and diffusion-weighted MRI in a patient with a recurrent solid pseudopapillary tumor of the pancreas. J Gastroenterol 2007; 42:918-922
[39] Arrigoni F, Calloni S, Huisman T, Chiapparini L. Conventional MRI. Handb Clin Neurol 2018; 154:219-234
[40] Watanabe Y, Okamoto K, Okada K, Aikawa M, Koyama I, Yamaguchi H. A case of aggressive solid pseudopapillary neoplasm: Comparison of clinical and pathologic features w ith non-aggressive cases. Pathol Int 2017; 67:202-207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, X., He, W., Huang, C. et al. Preoperative prediction of invasive behavior of pancreatic solid pseudopapillary neoplasm by MRI-based multiparametric radiomics models. Abdom Radiol 47, 3782–3791 (2022). https://doi.org/10.1007/s00261-022-03639-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03639-6