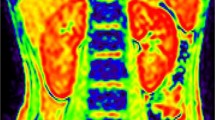
Abstract
Aim
Primary objective of this study was to compare R2* value of the post-stenotic kidney with contralateral kidney, kidneys of essential hypertensive patients, and healthy subjects using blood oxygen level-dependent magnetic resonance imaging (BOLD MRI) technique. The secondary objective was to study the effect of severity of stenosis and viability of kidneys on R2* value.
Methods
We compared 4 groups of kidneys including 92 with renal artery stenosis, 37 normal contralateral kidneys of unilateral renal artery stenosis patients, 62 kidneys of essential hypertensive patients, and 40 kidneys of healthy controls using BOLD MRI. Deoxyhemoglobin level represented by R2* was calculated before and after giving furosemide and was compared among different groups.
Results
Baseline means cortical R2* value did not differ between groups. Response to furosemide was reduced in stenotic kidneys as compared to essential hypertensive and healthy control groups (p < 0.001). The mean R2* value of the contralateral normal kidney group was not significantly different from the stenotic group. Baseline R2* value and delta R2* values did not differ between different degrees of stenosis. Higher mean cortical R2* was seen in stenotic kidneys which were small (< 7 cm) in size (24.27 ± 5.65 vs 21.7 ± 3.88; p value 0.02) or with poor corticomedullary differentiation (24.64 ± 5.8 vs 20.74 ± 3.34; p value 0.006) as compared to other stenotic kidneys. Similarly, the delta R2* value was also blunted in these small shrunken kidneys (p value < 0.001).
Conclusion
R2* values on BOLD MRI are significantly different between kidneys with and without renal artery stenosis and can potentially also predict the utility of revascularization.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001 Feb 8;344(6):431–42.
Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992 Jun;262(6 Pt 1):E763-778.
Nielsen K, Rehling M, Henriksen JH. Renal vein oxygen saturation in renal artery stenosis. Clin Physiol. 1992 Mar;12(2):179–84.
Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrology Dialysis Transplantation. 2010 Nov 1;25(11):3615–22.
Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol. 2006 May;290(5):F958-974.
[6] Zheng Z, Shi H, Ma H, Li F, Zhang J, Zhang Y. Renal oxygenation characteristics in healthy native kidneys: assessment with blood oxygen level-dependent magnetic resonance imaging. Nephron Physiol. 2014;128(3–4):47–54.
Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9868–72.
Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996 Dec 15;94(12):3271–5.
Piskunowicz M, Hofmann L, Zuercher E, Bassi I, Milani B, Stuber M, et al. A new technique with high reproducibility to estimate renal oxygenation using BOLD-MRI in chronic kidney disease. MagnReson Imaging. 2015 Apr;33(3):253–61.
Ebrahimi B, Gloviczki M, Woollard JR, Crane JA, Textor SC, Lerman LO. Compartmental analysis of renal BOLD MRI data: introduction and validation. Invest Radiol. 2012 Mar;47(3):175–82.
Pruijm M, Mendichovszky IA, Liss P, Van der Niepen P, Textor SC, Lerman LO, et al. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: a statement paper and systematic review. Nephrol Dial Transplant. 2018 01;33(suppl_2):ii22–8.
Pedersen M, Dissing TH, Mørkenborg J, Stødkilde-Jørgensen H, Hansen LH, Pedersen LB, et al. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005 Jun;67(6):2305–12.
Alford SK, Sadowski EA, Unal O, Polzin JA, Consigny DW, Korosec FR, et al. Detection of acute renal ischemia in swine using blood oxygen level-dependent magnetic resonance imaging. J Magn Reson Imaging. 2005 Sep;22(3):347–53.
Forster BB, MacKay AL, Whittall KP, Kiehl KA, Smith AM, Hare RD, et al. Functional magnetic resonance imaging: the basics of blood-oxygen-level dependent (Bold) imaging. Can Assoc Radiol J. 1998 Oct;49(5):320–9.
ASTRAL Investigators, Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009 Nov 12;361(20):1953–62.
Srivastava A, Hiralal, Chopra J, Sharma PK. A computed tomography (CT) evaluation of renal artery evaluation in healthy adult north Indian population. Int J Anat Res 2018;6:5207–5212.
Yamuna J, Chandrasekharan A, Rangasami R, Ramalakshmi S, Joseph S. Unenhanced renal magnetic resonance angiography in patients with chronic kidney disease & suspected renovascular hypertension: Can it affect patient management? Indian J Med Res. 2017 Nov;146(Supplement):S22–9.
Glodny B, Unterholzner V, Taferner B, Hofmann KJ, Rehder P, Strasak A, et al. Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urol. 2009 Dec 23;9:19.
Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994 Dec;267(6 Pt 2):F1059–1062.
Pruijm M, Hofmann L, Piskunowicz M, Muller M-E, Zweiacker C, Bassi I, et al. Determinants of renal tissue oxygenation as measured with BOLD-MRI in chronic kidney disease and hypertension in humans. PLoS One. 2014;9(4):e95895.
Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, et al. Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009 Sep;44(9):566–71.
Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012 Apr;81(7):684–9.
Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010 Apr;55(4):961–6.
Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009 Oct;297(4):F981-986.
[25] Wiecek A, Kokot F, Kuczera M, Grzeszczak W, Kiersztejn M. Plasma erythropoietin concentrations in renal venous blood of patients with unilateral renovascular hypertension. Nephrol Dial Transplant. 1992;7(3):221–4.
Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008 Nov;295(5):F1259-1270.
Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, et al. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension. 2011 Dec;58(6):1066–72.
Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008 Apr;19(4):780–8.
Djamali A, Sadowski EA, Samaniego-Picota M, Fain SB, Muehrer RJ, Alford SK, et al. Noninvasive assessment of early kidney allograft dysfunction by blood oxygen level-dependent magnetic resonance imaging. Transplantation. 2006 Sep 15;82(5):621–8.
Pruijm M, Hofmann L, Maillard M, Tremblay S, Glatz N, Wuerzner G, et al. Effect of sodium loading/depletion on renal oxygenation in young normotensive and hypertensive men. Hypertension. 2010 May;55(5):1116–22.
Stamey TA, Nudelman IJ, Good PH, Schwentker FN, Hendricks F. Functional characteristics of renovascular hypertension. Medicine (Baltimore). 1961 Dec;40:347–94.
Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001 Apr;16(4):765–70.
Herrmann SMS, Saad A, Eirin A, Woollard J, Tang H, McKusick MA, et al. Differences in GFR and tissue oxygenation, and interactions between stenotic and contralateral kidneys in unilateral atherosclerotic renovascular disease. Clin J Am Soc Nephrol. 2016 Mar 7;11(3):458–69.
Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014 Jan 2;370(1):13–22.
Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007 Dec;117(12):3810–20.
Christen T, Lemasson B, Pannetier N, Farion R, Remy C, Zaharchuk G, Barbier EL. Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology. 2012 Feb;262(2):495-502.
[37] He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med. 2007;57(1):115-126.
Niendorf T, Pohlmann A, Arakelyan K, Flemming B, Cantow K, Hentschel J, Grosenick D, Ladwig M, Reimann H, Klix S, Waiczies S, Seeliger E. How bold is blood oxygenation level-dependent (BOLD) magnetic resonance imaging of the kidney? Opportunities, challenges and future directions. Acta Physiol (Oxf). 2015 Jan;213(1):19-38.
Acknowledgements
None.
Funding
No funds, grants, or other support were received.
Author information
Authors and Affiliations
Contributions
Both HL and PS contributed equally to the manuscript and share the first authorship. This manuscript is a part of thesis done by PS and KP under supervision of HL. HL performed conceptualization; HL, PS, and RP contributed to methodology; PS and KP performed formal analysis and investigation; PS contributed to writing and preparation of original draft; PS, HL, RP, PY, PM, DB, SK, VA, AS, and SPS contributed to writing, reviewing, and editing of the manuscript; HL performed supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Ethics Committee of the Sanjay Gandhi Post-Graduate Institute of Medical Sciences, Lucknow, India (No. 2016–47-MD-EXP).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lal, H., Singh, P., Ponmalai, K. et al. Role of blood oxygen level-dependent magnetic resonance imaging in studying renal oxygenation changes in renal artery stenosis. Abdom Radiol 47, 1112–1123 (2022). https://doi.org/10.1007/s00261-022-03408-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03408-5