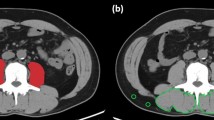
Abstract
Purpose
Nutrition is an important outcome predictor in oncology patients including treatment response, physical disability, quality of life, and overall survival. Sarcopenia (loss of skeletal muscle mass and function) is a demonstrated marker of nutritional status in adults, but data are more limited in children. The purpose of this study was to evaluate whether total psoas muscle area (tPMA) measured at the time of cancer diagnosis predicts overall survival (OS), disease free survival (DFS), or number of days neutropenic.
Methods
A retrospective study was performed. tPMA was measured at the L3 and L4 mid-lumbar vertebral body level by a single reviewer on cross-sectional imaging studies performed within 2 weeks of primary oncologic diagnosis for all oncology patients who received their primary therapy at Cincinnati Children’s Hospital between 1/1/2000 and 12/31/2013. Spearman’s correlation was used to assess the association between tPMA and OS, DFS, days neutropenic, and adjusted days neutropenic. Subanalysis was performed assessing the relationship of tumor type and age at diagnosis with each parameter.
Results
164 patients (median age 9.9 years; 89 M/75 F) were included in the study. Days neutropenic and normalized days neutropenic were significantly but weakly negatively correlated with tPMA at L3 (r = − 0.24, p < 0.002 and r = − 0.18, p < 0.05 respectively) and L4 (r = − 0.25, p < 0.002; and and r = − 0.19, p < 0.02 respectively). At subanalysis, the correlation between anthropometric features and normalized days neutropenic was only seen with brain tumors. There was no statistically significant relationship between sarcopenia at diagnosis and DFS or OS overall or in subanalysis.
Conclusion
There is a weak inverse relationship between days neutropenic and psoas muscle bulk in pediatric and young adult oncology patients suggesting a relationship between nutritional status and cell recovery. Measures of sarcopenia, however, did not correlate with DFS or OS.
Graphic abstract



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, AJT, upon reasonable request.
References
Bauer J, Jurgens H, Fruhwald MC (2011) Important aspects of nutrition in children with cancer. Adv Nutr 2 (2):67-77, https://doi.org/10.3945/an.110.000141.
Santilli V, Bernetti A, Mangone M, Paoloni M (2014) Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 11 (3):177-180
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P (2017) ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 36 (1):49-64, https://doi.org/10.1016/j.clnu.2016.09.004.
Gilligan LA, Towbin AJ, Dillman JR, Somasundaram E, Trout AT (2020) Quantification of skeletal muscle mass: sarcopenia as a marker of overall health in children and adults. Pediatric radiology 50 (4):455-464, https://doi.org/10.1007/s00247-019-04562-7.
Pamoukdjian F, Bouillet T, Levy V, Soussan M, Zelek L, Paillaud E (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr 37 (4):1101-1113, https://doi.org/10.1016/j.clnu.2017.07.010.
Morrell GR, Ikizler TA, Chen X, Heilbrun ME, Wei G, Boucher R, Beddhu S (2016) Psoas Muscle Cross-sectional Area as a Measure of Whole-body Lean Muscle Mass in Maintenance Hemodialysis Patients. J Ren Nutr 26 (4):258-264, https://doi.org/10.1053/j.jrn.2016.02.002.
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. European journal of cancer (Oxford, England : 1990) 57:58–67, https://doi.org/10.1016/j.ejca.2015.12.030.
Rayar M, Webber CE, Nayiager T, Sala A, Barr RD (2013) Sarcopenia in children with acute lymphoblastic leukemia. Journal of pediatric hematology/oncology 35 (2):98-102, https://doi.org/10.1097/MPH.0b013e318279eea2.
Hawkins RB, Mehaffey JH, Charles EJ, Kern JA, Lim DS, Teman NR, Ailawadi G (2018) Psoas Muscle Size Predicts Risk-Adjusted Outcomes After Surgical Aortic Valve Replacement. Ann Thorac Surg 106 (1):39-45, https://doi.org/10.1016/j.athoracsur.2018.02.010.
Mosteller RD (1987) Simplified calculation of body-surface area. The New England journal of medicine 317 (17):1098, https://doi.org/10.1056/NEJM198710223171717.
Lurz E, Patel H, Lebovic G, Quammie C, Woolfson JP, Perez M, Ricciuto A, Wales PW, Kamath BM, Chavhan GB, Juni P, Ng VL (2020) Paediatric reference values for total psoas muscle area. J Cachexia Sarcopenia Muscle 11 (2):405-414, https://doi.org/10.1002/jcsm.12514.
Phillips B, Wade R, Stewart LA, Sutton AJ (2010) Systematic review and meta-analysis of the discriminatory performance of risk prediction rules in febrile neutropaenic episodes in children and young people. European journal of cancer (Oxford, England : 1990) 46 (16):2950–2964, https://doi.org/10.1016/j.ejca.2010.05.024.
Cumbo TA, Segal BH (2004) Prevention, diagnosis, and treatment of invasive fungal infections in patients with cancer and neutropenia. J Natl Compr Canc Netw 2 (5):455-469, https://doi.org/10.6004/jnccn.2004.0036.
Fisher BT, Robinson PD, Lehrnbecher T, Steinbach WJ, Zaoutis TE, Phillips B, Sung L (2018) Risk Factors for Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review. J Pediatric Infect Dis Soc 7 (3):191-198, https://doi.org/10.1093/jpids/pix030.
Sun Y, Huang H, Chen J, Li J, Ma J, Li J, Liang Y, Wang J, Li Y, Yu K, Hu J, Jin J, Wang C, Wu D, Xiao Y, Huang X (2015) Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: a multicenter, prospective, observational study in China. Tumour Biol 36 (2):757-767, https://doi.org/10.1007/s13277-014-2649-7.
Suzuki D, Kobayashi R, Sano H, Hori D, Kobayashi K (2018) Sarcopenia after induction therapy in childhood acute lymphoblastic leukemia: its clinical significance. International journal of hematology 107 (4):486-489, https://doi.org/10.1007/s12185-017-2388-9.
Conner JM, Aviles-Robles MJ, Asdahl PH, Zhang FF, Ojha RP (2016) Malnourishment and length of hospital stay among paediatric cancer patients with febrile neutropaenia: a developing country perspective. BMJ Support Palliat Care 6 (3):338-343, https://doi.org/10.1136/bmjspcare-2015-001020.
Israels T, van de Wetering MD, Hesseling P, van Geloven N, Caron HN, Molyneux EM (2009) Malnutrition and neutropenia in children treated for Burkitt lymphoma in Malawi. Pediatric blood & cancer 53 (1):47-52, https://doi.org/10.1002/pbc.22032.
Shah S, Rahman MA, Mannan MA (2012) Nutritional parameters in children with cancer. Mymensingh Med J 21 (3):522-528
Triarico S, Rinninella E, Cintoni M, Capozza MA, Mastrangelo S, Mele MC, Ruggiero A (2019) Impact of malnutrition on survival and infections among pediatric patients with cancer: a retrospective study. Eur Rev Med Pharmacol Sci 23 (3):1165-1175, https://doi.org/10.26355/eurrev_201901_17009.
Kawakubo N, Kinoshita Y, Souzaki R, Koga Y, Oba U, Ohga S, Taguchi T (2019) The Influence of Sarcopenia on High-Risk Neuroblastoma. J Surg Res 236:101-105, https://doi.org/10.1016/j.jss.2018.10.048.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older P (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39 (4):412-423, https://doi.org/10.1093/ageing/afq034.
Springer J, Springer JI, Anker SD (2017) Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail 4 (4):492-498, https://doi.org/10.1002/ehf2.12237.
Moorthi RN, Avin KG (2017) Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 26 (3):219-228, https://doi.org/10.1097/MNH.0000000000000318.
Chang KV, Hsu TH, Wu WT, Huang KC, Han DS (2016) Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc 17 (12):1164 e1167–1164 e1115, https://doi.org/10.1016/j.jamda.2016.09.013.
Kim G, Kang SH, Kim MY, Baik SK (2017) Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PloS one 12 (10):e0186990, https://doi.org/10.1371/journal.pone.0186990.
Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ (2017) Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis 12:669-675, https://doi.org/10.2147/COPD.S130790.
Achim V, Bash J, Mowery A, Guimaraes AR, Li R, Schindler J, Wax M, Andersen P, Clayburgh D (2017) Prognostic Indication of Sarcopenia for Wound Complication After Total Laryngectomy. JAMA Otolaryngol Head Neck Surg 143 (12):1159-1165, https://doi.org/10.1001/jamaoto.2017.0547.
Woo J (2017) Sarcopenia. Clin Geriatr Med 33 (3):305-314, https://doi.org/10.1016/j.cger.2017.02.003.
Lurz E, Patel H, Frimpong RG, Ricciuto A, Kehar M, Wales PW, Towbin AJ, Chavhan GB, Kamath BM, Ng VL (2018) Sarcopenia in Children With End-Stage Liver Disease. Journal of pediatric gastroenterology and nutrition 66 (2):222-226, https://doi.org/10.1097/MPG.0000000000001792.
Lopez JJ, Cooper JN, Albert B, Adler B, King D, Minneci PC (2017) Sarcopenia in children with perforated appendicitis. J Surg Res 220:1-510. https://doi.org/10.1016/j.jss.2017.05.059.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MM and BZ. The first draft of the manuscript was written by MM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Andrew T. Trout Grant funding: Siemens Medical Solutions; Canon Medical Systems, Research support: Perspectum, Honoraria for authorship: Elsevier; Wolters-Kluwer. Alexander J. Towbin—Grant funding: Guerbet; Cystic Fibrosis Foundation, Unpaid advisory board member: IBM Watson Health, KLAS, Consultant: Applied Radiology, Author royalties: Elsevier. Morgan P. McBee and Cody Woodhouse, James I. Geller, Ethan A. Smith and Bin Zhang declares that they have no conflict of interest.
Ethical approval
This retrospective HIPAA compliant study was approved by the institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McBee, M.P., Woodhouse, C., Trout, A.T. et al. Skeletal muscle mass as a marker to predict outcomes in children and young adults with cancer. Abdom Radiol 47, 452–459 (2022). https://doi.org/10.1007/s00261-021-03301-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03301-7