Abstract
Purpose
Hepatic surface nodularity quantified on CT images has shown promising results in staging hepatic fibrosis in chronic hepatitis C. The aim of this study was to evaluate hepatic surface nodularity, serum fibrosis indices, and a linear combination of them for staging fibrosis in chronic liver disease, mainly chronic hepatitis B.
Methods
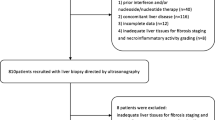
We developed a semiautomated software quantifying hepatic surface nodularity on CT images. Hepatic surface nodularity and serum fibrosis indices were assessed in the development group of 125 patients to generate 3 linear models combining hepatic surface nodularity with the aspartate aminotransferase to platelet ratio index, fibrosis-4 index, or platelet count in reference to the METAVIR scoring system. The models were validated in 183 patients.
Results
Hepatic surface nodularity and serum fibrosis indices all significantly correlated with fibrosis stages. For binary classifications into cirrhosis (F4), advanced fibrosis (≥ F3), and significant fibrosis (≥ F2), hepatic surface nodularity was significantly different across categories. The areas under the curve (AUCs) of the best model were 0.901, 0.872, and 0.794 for cirrhosis, advanced fibrosis, and significant fibrosis, respectively, higher than serum fibrosis indices alone (0.797–0.802, 0.799–0.818, and 0.761–0.773). In the validation group, the same model likewise showed higher AUCs (0.872, 0.831, and 0.850) compared to serum fibrosis indices (0.722–0.776, 0.692–0.768, and 0.695–0.769; p < 0.001 for F4).
Conclusion
Hepatic surface nodularity combined with serum blood test could be a practical method to predict cirrhosis, advanced fibrosis, and significant fibrosis in chronic liver disease patients, providing higher accuracy than using serum fibrosis indices alone.



Similar content being viewed by others
References
Trautwein C, Friedman SL, Schuppan D, Pinzani M (2015) Hepatic fibrosis: Concept to treatment. J Hepatol 62:S15–24. https://doi.org/10.1016/j.jhep.2015.02.039
Schiff ER, Lee SS, Chao YC, et al. (2011) Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol 9:274–276. https://doi.org/10.1016/j.cgh.2010.11.040
Schuppan D, Kim YO (2013) Evolving therapies for liver fibrosis. J Clin Invest 123:1887–1901. https://doi.org/10.1172/jci66028
Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344:495–500. https://doi.org/10.1056/nejm200102153440706
Bedossa P, Dargère D, Paradis V (2003) Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology (Baltimore, Md) 38:1449–1457. https://doi.org/10.1016/j.hep.2003.09.022
Wai CT, Greenson JK, Fontana RJ, et al. (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology (Baltimore, Md) 38:518–526. https://doi.org/10.1053/jhep.2003.50346
Sterling RK, Lissen E, Clumeck N, et al. (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md) 43:1317–1325. https://doi.org/10.1002/hep.21178
Cross TJ, Rizzi P, Berry PA, et al. (2009) King’s Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol 21:730–738. https://doi.org/10.1097/MEG.0b013e32830dfcb3
Gaia S, Carenzi S, Barilli AL, et al. (2011) Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol 54:64–71. https://doi.org/10.1016/j.jhep.2010.06.022
Gao Y, Zheng J, Liang P, et al. (2018) Liver Fibrosis with Two-dimensional US Shear-Wave Elastography in Participants with Chronic Hepatitis B: A Prospective Multicenter Study. Radiology 289:407–415. https://doi.org/10.1148/radiol.2018172479
Yin M, Talwalkar JA, Glaser KJ, et al. (2007) Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 5:1207–1213.e1202. https://doi.org/10.1016/j.cgh.2007.06.012
Zhang W, Zhu Y, Zhang C, Ran H (2019) Diagnostic Accuracy of 2-Dimensional Shear Wave Elastography for the Staging of Liver Fibrosis: A Meta-analysis. J Ultrasound Med 38:733–740. https://doi.org/10.1002/jum.14760
Herrmann E, de Lédinghen V, Cassinotto C, et al. (2018) Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology (Baltimore, Md) 67:260–272. https://doi.org/10.1002/hep.29179
Friedrich-Rust M, Ong MF, Martens S, et al. (2008) Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 134:960–974. https://doi.org/10.1053/j.gastro.2008.01.034
Singh S, Venkatesh SK, Wang Z, et al. (2015) Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 13:440–451.e446. https://doi.org/10.1016/j.cgh.2014.09.046
Smith AD, Porter KK, Elkassem AA, Sanyal R, Lockhart ME. (2019) Current Imaging Techniques for Noninvasive Staging of Hepatic Fibrosis. AJR American journal of roentgenology 213:77–89. https://doi.org/10.2214/ajr.19.21144
Smith AD, Branch CR, Zand K, et al. (2016) Liver Surface Nodularity Quantification from Routine CT Images as a Biomarker for Detection and Evaluation of Cirrhosis. Radiology 280:771–781. https://doi.org/10.1148/radiol.2016151542
Pickhardt PJ, Malecki K, Kloke J, Lubner MG (2016) Accuracy of Liver Surface Nodularity Quantification on MDCT as a Noninvasive Biomarker for Staging Hepatic Fibrosis. AJR American journal of roentgenology 207:1194–1199. https://doi.org/10.2214/ajr.16.16514
Lo GC, Besa C, King MJ, et al. (2017) Feasibility and reproducibility of liver surface nodularity quantification for the assessment of liver cirrhosis using CT and MRI. European journal of radiology open 4:95–100. https://doi.org/10.1016/j.ejro.2017.07.001
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology (Baltimore, Md) 24:289–293. https://doi.org/10.1002/hep.510240201
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Moon KM, Kim G, Baik SK, et al. (2013) Ultrasonographic scoring system score versus liver stiffness measurement in prediction of cirrhosis. Clin Mol Hepatol 19:389–398. https://doi.org/10.3350/cmh.2013.19.4.389
Goshima S, Kanematsu M, Kondo H, et al. (2015) Computer-aided assessment of hepatic contour abnormalities as an imaging biomarker for the prediction of hepatocellular carcinoma development in patients with chronic hepatitis C. European journal of radiology 84:811–815. https://doi.org/10.1016/j.ejrad.2015.01.009
Goshima S, Kanematsu M, Kobayashi T, et al. (2012) Staging hepatic fibrosis: computer-aided analysis of hepatic contours on gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced hepatocyte-phase magnetic resonance imaging. Hepatology (Baltimore, Md) 55:328–329. https://doi.org/10.1002/hep.24677
Kim SW, Kim YR, Choi KH, Cho EY, Song JS, Kim JE, et al. (2020) Staging of Liver Fibrosis by Means of Semiautomatic Measurement of Liver Surface Nodularity in MRI. AJR American journal of roentgenology 215:624–630. https://doi.org/10.2214/AJR.19.22041
Marcellin P, Ziol M, Bedossa P, et al. (2009) Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 29:242–247. https://doi.org/10.1111/j.1478-3231.2008.01802.x
Rossi E, Adams LA, Bulsara M, Jeffrey GP (2007) Assessing liver fibrosis with serum marker models. Clin Biochem Rev 28:3–10
Castéra L, Vergniol J, Foucher J, et al. (2005) Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128:343–350. https://doi.org/10.1053/j.gastro.2004.11.018
Liu Y, Dong CF, Yang G, Liu J, Yao S, Li HY, et al. (2015) Optimal linear combination of ARFI, transient elastography and APRI for the assessment of fibrosis in chronic hepatitis B. Liver Int 35:816-825. https://doi.org/10.1111/liv.12564
Boursier J, Vergniol J, Sawadogo A, et al. (2009) The combination of a blood test and Fibroscan improves the non-invasive diagnosis of liver fibrosis. Liver Int 29:1507–1515. https://doi.org/10.1111/j.1478-3231.2009.02101.x
Goshima S, Bae KT (2017) Liver Surface Nodularity as a Biomarker for Detection and Evaluation of Cirrhosis. Radiology 283:921–922. https://doi.org/10.1148/radiol.2017170112
Sartoris R, Rautou PE, Elkrief L, et al. (2018) Quantification of Liver Surface Nodularity at CT: Utility for Detection of Portal Hypertension. Radiology 289:698–707. https://doi.org/10.1148/radiol.2018181131
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B5076568). The receiver of the fund is Bohyun Kim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interests.
Ethical approval
Informed consent was waived by the institutional IRB due to retrospective analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cho, H.J., Choi, J., Kim, B. et al. Combining hepatic surface nodularity and serum tests better predicts hepatic fibrosis stages in chronic liver disease. Abdom Radiol 46, 4189–4199 (2021). https://doi.org/10.1007/s00261-021-03113-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03113-9