Abstract
Purpose
The purpose of this study is to analyze trends in Medicare volume and physician reimbursement for percutaneous ablation, surgical ablation, and resection of liver tumors from 2010 to 2018.
Methods
Claims from the Medicare Part B PSPSMF for the years 2010 to 2018 were extracted using the CPT codes for percutaneous and surgical ablation of liver tumors and surgical liver resection. Total procedural volume and physician payment were analyzed by procedure and physician specialty.
Results
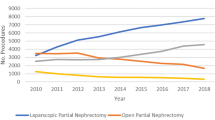
From 2010 to 2018, the volume of percutaneous ablation of liver tumors increased 94.3% from 1630 to 3168 procedures, and the volume of surgical ablations increased 86.2% from 593 to 1104 procedures. In contrast, there was a 16.8% decrease in liver resections from 10,807 to 8994 procedures. Physician reimbursement for percutaneous ablation decreased from $702.41 to $610.11 (− 13.1%). Conversely, reimbursement for resection increased from $849.18 to $1015.06 (19.5%). Reimbursement for surgical ablation also increased from $722.36 to $744.25 (3.0%). In 2018, physician reimbursement for resection and surgical ablation were 66% and 22% more than that for percutaneous ablation.
Conclusion
An increasing number of patients with liver tumors were treated with percutaneous ablation from 2010 to 2018. Despite higher morbidity, a dwindling set of theoretical advantages over percutaneous ablation, and higher overall costs, the volume of surgical ablation also increased over this time period. The findings of this study suggest that a reevaluation of practice and referral patterns for surgical ablation of liver tumors is warranted in many institutions.




Similar content being viewed by others
Data availability
Publicly available data obtained from Medicare were used.
References
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301-1314.
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329-38.
Llovet JM, Fuster J, Bruix J, Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S115-20.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
Tsilimigras DI, Bagante F, Sahara K, Moris D, Hyer JM, Wu L, Ratti F, Marques HP, Soubrane O, Paredes AZ, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Prognosis After Resection of Barcelona Clinic Liver Cancer (BCLC) Stage 0, A, and B Hepatocellular Carcinoma: A Comprehensive Assessment of the Current BCLC Classification. Ann Surg Oncol. 2019;26(11):3693-3700.
Xu Y, Shen Q, Liu P, Xu Z, Wu P, Lu Z, Chen Y, Huang B, Qian G. Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy and long-term outcomes. Eur Radiol. 2017;27(9):3877-3887.
Yang G1, Xiong Y2, Sun J1, Wang G3, Li W1, Tang T1, Li J4. The efficacy of microwave ablation versus liver resection in the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Int J Surg. 2020.
Hinshaw JL, Lubner, MG, Ziemlewicz TJ, Lee FT, Brace CL. Percutaneous Tumor Ablation Tools: Microwave, Radiofrequency, or Cryoablation—What Should You Use and Why? RadioGraphics 2014; 35:1344–1362.
Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, Liu GJ, Liang JY, Lau WY. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115(9):1914-23.
Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol 2013;25(2):187–194.
Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31(4):426–432.
Warlick CA, Lima GC, Allaf ME, et al. Clinical sequelae of radiographic iceball involvement of collecting system during computed tomography–guided percutaneous renal tumor cryoablation. Urology 2006;67(5): 91.
Janzen NK, Perry KT, Han KR, et al. The effects of intentional cryoablation and radio frequency ablation of renal tissue involving the collecting system in a porcine model. J Urol 2005;173(4):1368–1374.
Massarweh NN1, Park JO, Farjah F, Yeung RS, Symons RG, Vaughan TL, Baldwin LM, Flum DR. Trends in the utilization and impact of radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2010;210(4):441-8.
Giglio MC, Logghe B, Garofalo E, Tomassini F, Vanlander A, Berardi G, Montalti R, Troisi RI. Laparoscopic Versus Open Thermal Ablation of Colorectal Liver Metastases: A Propensity Score-Based Analysis of Local Control of the Ablated Tumors. Ann Surg Oncol. 2020.
Puijk RS, Ruarus AH, Vroomen LGPH, van Tilborg AAJM, Scheffer HJ, Nielsen K, de Jong MC, de Vries JJJ, Zonderhuis BM, Eker HH, Kazemier G, Verheul H, van der Meijs BB, van Dam L, Sorgedrager N, Coupé VMH, van den Tol PMP, Meijerink MR; COLLISION Trial Group. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18(1):821.
Cassera MA, Potter KW, Ujiki MB, Swanström LL, Hansen PD. Computed tomography (CT)-guided versus laparoscopic radiofrequency ablation: a single-institution comparison of morbidity rates and hospital costs. Surg Endosc. 2011;25(4):1088-1095.
Yang JD, Luu M, Singal AG, et al. Factors Associated With Detection and Survival of T1 Hepatocellular Carcinoma in the United States: National Cancer Database Analysis. J Natl Compr Canc Netw. 2020;18(9):1210-1220.
Harvey L. Neiman Health Policy Institute Medicare Beneficiary Enrollment Tool. <https://neimanhpi.org/medicare-beneficiary-enrollment-tool>. Accessed 4/13/2020.
Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300-307.
Wang T1, Zhang XY2, Lu X3, Zhai B1. Laparoscopic Microwave Ablation of Hepatocellular Carcinoma at Liver Surface: Technique Effectiveness and Long-Term Outcomes. Technol Cancer Res Treat. 2019;18:1533033818824338.
Hsiao WC1, Braun P, Yntema D, Becker ER. Estimating physicians’ work for a resource-based relative-value scale. N Engl J Med. 1988;319(13):835-41.
Piccioni F, Poli A, Templeton LC, Templeton TW, Rispoli M, Vetrugno L, Santonastaso D, Valenza F. Anesthesia for Percutaneous Radiofrequency Tumor Ablation (PRFA): A Review of Current Practice and Techniques. Local Reg Anesth. 2019;12:127-137.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors had a meaningful contribution to the design of the study and writing/editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Ethical approval
IRB approval was waived because only publicly available data were used.
Consent to participate
Only publicly available data were used.
Consent for publication
All authors consent to the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lindquester, W.S., Dhangana, R., Pinter, J. et al. Percutaneous ablation versus surgical ablation and resection of liver tumors: medicare volume and physician reimbursement trends from 2010 to 2018. Abdom Radiol 46, 4056–4061 (2021). https://doi.org/10.1007/s00261-021-03054-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03054-3