Abstract
Objectives
To develop and validate a nomogram for the preoperative prediction of pancreatic serous cystic neoplasm (SCN) and mucinous cystic neoplasm (MCN) based on multidetector computed tomography (MDCT).
Materials and methods
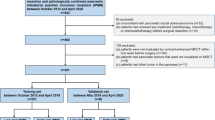
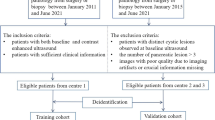
In this retrospective study, the data of 227 patients with SCN and MCN were analyzed. Each patient underwent MDCT and surgical resection. A multivariable logistic regression model was developed using a training set consisting of 129 patients with SCN and 38 patients with MCN who were admitted between October 2012 and April 2019. The model was validated in 60 consecutive patients, 44 of whom had SCN and 16 of whom had MCN, admitted between May 2019 and April 2020. The regression model was adopted to establish a nomogram. Nomogram performance was determined by its discriminative ability and clinical utility.
Result
The multivariable logistic regression model included sex, size, location, shape, cyst characteristic, and cystic wall thickening. The individualized prediction nomogram showed good discrimination in the training sample (AUC 0.89; 95% CI 0.83–0.95) and in the validation sample (AUC 0.81; 95% CI 0.70–0.94). If the threshold probability is between 0.03 and 0.9, and > 0.93 in the prediction model, using the nomogram to predict SCN and MCN is more beneficial than the treat-all-patients as SCN scheme or the treat-all-patients as MCN scheme. The prediction model showed better discrimination than the radiologists’ diagnosis (AUC = 0.68).
Conclusion
The nomogram could predict SCN and MCN preoperatively and may aid clinical decision-making.





Similar content being viewed by others
References
Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013; 45:703-711
J AM, Masashi F, S KD, Yin LAK, D NI, D OR. WHO Classification of Tumours of the Digestive System, 5 ed. Lyon, France: IARC Press, 2019
Goh BK, Tan DM, Thng CH, et al. Are the Sendai and Fukuoka consensus guidelines for cystic mucinous neoplasms of the pancreas useful in the initial triage of all suspected pancreatic cystic neoplasms? A single-institution experience with 317 surgically-treated patients. Ann Surg Oncol 2014; 21:1919-1926
Kim SY, Lee JM, Kim SH, et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol 2006; 187:1192-1198
Levy P, Rebours V. The Role of Endoscopic Ultrasound in the Diagnosis of Cystic Lesions of the Pancreas. Visc Med 2018; 34:192-196
Ngamruengphong S, Lennon AM. Analysis of Pancreatic Cyst Fluid. Surg Pathol Clin 2016; 9:677-684
Burk KS, Knipp D, Sahani DV. Cystic Pancreatic Tumors. Magn Reson Imaging Clin N Am 2018; 26:405-420
Cho CS, Russ AJ, Loeffler AG, et al. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol 2013; 20:3112-3119
Cohen-Scali F, Vilgrain V, Brancatelli G, et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology 2003; 228:727-733
Lee JH, Kim JK, Kim TH, et al. MRI features of serous oligocystic adenoma of the pancreas: differentiation from mucinous cystic neoplasm of the pancreas. Br J Radiol 2012; 85:571-576
Pozzessere C, Castanos Gutierrez SL, Corona-Villalobos CP, et al. Diffusion-Weighted Magnetic Resonance Imaging in Distinguishing Between Mucin-Producing and Serous Pancreatic Cysts. J Comput Assist Tomogr 2016; 40:505-512
Pandey P, Pandey A, Shao N, et al. Added value of apparent diffusion coefficient in distinguishing between serous and mucin-producing pancreatic cystic neoplasms. Eur Radiol 2019; 29:4660-4669
Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho 2009; 36:2495–2501
Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol 2000; 175:99-103
Yang J, Guo X, Zhang H, et al. Differential diagnosis of pancreatic serous cystadenoma and mucinous cystadenoma: utility of textural features in combination with morphological characteristics. BMC cancer 2019; 19:1223
Yang J, Guo X, Ou X, Zhang W, Ma X. Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Frontiers in oncology 2019; 9:494
Wei R, Lin K, Yan W, et al. Computer-Aided Diagnosis of Pancreas Serous Cystic Neoplasms: A Radiomics Method on Preoperative MDCT Images. Technol Cancer Res Treat 2019; 18:1533033818824339
Xie H, Ma S, Guo X, Zhang X, Wang X. Preoperative differentiation of pancreatic mucinous cystic neoplasm from macrocystic serous cystic adenoma using radiomics: Preliminary findings and comparison with radiological model. Eur J Radiol 2020; 122:108747
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BJOG 2015; 122:434-443
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006; 26:565-574
Funding
This work was supported in part by the National Science Foundation for Scientists of China (81871352), Clinical Research Plan of SHDC (SHDC2020CR4073), 234 Platform Discipline Consolidation Foundation Project (2019YPT001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shao, C., Feng, X., Yu, J. et al. A nomogram for predicting pancreatic mucinous cystic neoplasm and serous cystic neoplasm. Abdom Radiol 46, 3963–3973 (2021). https://doi.org/10.1007/s00261-021-03038-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03038-3