Abstract
Purpose
To evaluate safety and efficacy of radiation segmentectomy (RS) with 90Y glass microspheres in patients with limited metastatic liver disease not amenable to resection or percutaneous ablation.
Methods
Patients with ≤ 3 tumors treated with RS from 6/2015 to 12/2017 were included. Target tumor radiation dose was > 190 Gy based on medical internal radiation dose (MIRD) dosimetry. Tumor response, local tumor progression (LTP), LTP-free survival (LTPFS) and disease progression rate in the treated segment were defined using Choi and RECIST 1.1 criteria. Toxicities were evaluated using modified SIR criteria.
Results
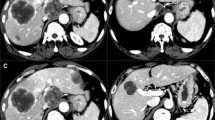
Ten patients with 14 tumors underwent 12 RS. Median tumor size was 3 cm (range 1.4–5.6). Median follow-up was 17.8 months (range 1.6–37.3). Response rates per Choi and RECIST 1.1 criteria were 8/8 (100%) and 4/9 (44%), respectively. Overall LTP rate was 3/14 (21%) during the study period. One-, two- and three-year LTPFS was 83%, 83% and 69%, respectively. Median LTPFS was not reached. Disease progression rate in the treated segment was 6/18 (33%). Median overall survival was 41.5 months (IQR 16.7–41.5). Median delivered tumor radiation dose was 293 Gy (range 163–1303). One major complication was recorded in a patient post-Whipple procedure who suffered anaphylactic reaction to prophylactic cefotetan and liver abscess in RS region 6.5 months post-RS. All patients were alive on last follow-up.
Conclusion
RS of ≤ 3 hepatic segments can safely provide a 2-year local tumor control rate of 83% in selected patients with limited metastatic liver disease and limited treatment options. Optimal dosimetry methodology requires further investigation.




Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Raw data were generated at Memorial Sloan Kettering Cancer Center. Derived data supporting the findings of this study are available from the corresponding author C.T. Sofocleous on request.
References
Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79(1):163-71.
Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60(1):192-201.
Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018:171768.
Biederman DM, Titano JJ, Bishay VL, Durrani RJ, Dayan E, Tabori N, et al. Radiation Segmentectomy versus TACE Combined with Microwave Ablation for Unresectable Solitary Hepatocellular Carcinoma Up to 3 cm: A Propensity Score Matching Study. Radiology. 2017;283(3):895-905.
Kurilova I, Bendet A, Petre EN, Boas FE, Kaye E, Gonen M, et al. Factors Associated With Local Tumor Control and Complications After Thermal Ablation of Colorectal Cancer Liver Metastases: A 15-year Retrospective Cohort Study. Clin Colorectal Cancer. 2020.
Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J Vasc Interv Radiol. 2018;29(2):268-75.e1.
Kurilova I, Beets-Tan RGH, Flynn J, Gonen M, Ulaner G, Petre EN, et al. Factors Affecting Oncologic Outcomes of 90Y Radioembolization of Heavily Pre-Treated Patients With Colon Cancer Liver Metastases. Clin Colorectal Cancer. 2019;18(1):8-18.
Boas FE, Bodei L, Sofocleous CT. Radioembolization of Colorectal Liver Metastases: Indications, Technique, and Outcomes. J Nucl Med. 2017;58(Suppl 2):104s-11s.
Padia SA, Kwan SW, Roudsari B, Monsky WL, Coveler A, Harris WP. Superselective yttrium-90 radioembolization for hepatocellular carcinoma yields high response rates with minimal toxicity. Journal of vascular and interventional radiology : JVIR. 2014;25(7):1067-73.
Sofocleous CT, Boas FE. Radiation Segmentectomy for Hepatocellular Carcinoma: Ready for Prime Time? Radiology. 2018:180163.
Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151(6):1155-63.e2.
Padia SA, Johnson GE, Horton KJ, Ingraham CR, Kogut MJ, Kwan S, et al. Segmental Yttrium-90 Radioembolization versus Segmental Chemoembolization for Localized Hepatocellular Carcinoma: Results of a Single-Center, Retrospective, Propensity Score-Matched Study. Journal of vascular and interventional radiology : JVIR. 2017;28(6):777-85.e1.
Biederman DM, Titano JJ, Korff RA, Fischman AM, Patel RS, Nowakowski FS, et al. Radiation Segmentectomy versus Selective Chemoembolization in the Treatment of Early-Stage Hepatocellular Carcinoma. Journal of vascular and interventional radiology : JVIR. 2018;29(1):30-7.e2.
Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018;287(3):1050-8.
Burrill J. HU, Liu D.M. Advances in radioembolization - Embolics and isotopes. Nuclear Medi Radiat Ther. (2011;2:107).
Lewandowski RJ, Thurston KG, Goin JE, Wong CY, Gates VL, Van Buskirk M, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol. 2005;16(12):1641-51.
Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst. 2017;109(9).
Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163(6):1238-44.
Meiers C, Taylor A, Geller B, Toskich B. Safety and initial efficacy of radiation segmentectomy for the treatment of hepatic metastases. J Gastrointest Oncol. 2018;9(2):311-5.
Padia SA, Johnson GE, Agopian VG, DiNorcia J, Srinivasa RN, Sayre J, et al. Yttrium-90 radiation segmentectomy for hepatic metastases: A multi-institutional study of safety and efficacy. J Surg Oncol. 2020.
Crane CH, Koay EJ. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer. 2016;122(13):1974-86.
Weng Z, Ertle J, Zheng S, Lauenstein T, Mueller S, Bockisch A, et al. Choi criteria are superior in evaluating tumor response in patients treated with transarterial radioembolization for hepatocellular carcinoma. Oncol Lett. 2013;6(6):1707-12.
Jongen JMJ, Rosenbaum C, Braat M, van den Bosch M, Sze DY, Kranenburg O, et al. Anatomic versus Metabolic Tumor Response Assessment after Radioembolization Treatment. Journal of vascular and interventional radiology : JVIR. 2018;29(2):244-53.e2.
Shady W, Kishore S, Gavane S, Do RK, Osborne JR, Ulaner GA, et al. Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after (90)Y radioembolization of colorectal liver metastases: A comparison with SUVmax, SUVpeak, and RECIST 1.0. European journal of radiology. 2016;85(6):1224-31.
Shady W, Sotirchos VS, Do RK, Pandit-Taskar N, Carrasquillo JA, Gonen M, et al. Surrogate Imaging Biomarkers of Response of Colorectal Liver Metastases After Salvage Radioembolization Using 90Y-Loaded Resin Microspheres. AJR Am J Roentgenol. 2016;207(3):661-70.
Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. Journal of vascular and interventional radiology : JVIR. 2003;14(9 Pt 2):S199-202.
Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al. Research reporting standards for radioembolization of hepatic malignancies. Journal of vascular and interventional radiology : JVIR. 2011;22(3):265-78.
Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: The MIRD equations for dose calculations. J Nucl Med. 2006;47(7):1209-11.
Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. Journal of vascular and interventional radiology : JVIR. 2006;17(8):1251-78.
He X, Zhang P, Li Z, Bi F, Xu F, Wang X, et al. Curative-intent radiotherapy in patients with oligometastatic lesions from colorectal cancer: A single-center study. Medicine. 2018;97(40):e12601.
Dupre A, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ, Malik HZ. Curative-intent treatment of recurrent colorectal liver metastases: A comparison between ablation and resection. Eur J Surg Oncol. 2017;43(10):1901-7.
Hickey R, Lewandowski RJ, Prudhomme T, Ehrenwald E, Baigorri B, Critchfield J, et al. 90Y Radioembolization of Colorectal Hepatic Metastases Using Glass Microspheres: Safety and Survival Outcomes from a 531-Patient Multicenter Study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57(5):665-71.
Sofocleous CT, Violari EG, Sotirchos VS, Shady W, Gonen M, Pandit-Taskar N, et al. Radioembolization as a Salvage Therapy for Heavily Pretreated Patients With Colorectal Cancer Liver Metastases: Factors That Affect Outcomes. Clin Colorectal Cancer. 2015;14(4):296-305.
Kurilova I, Beets-Tan RGH, Ulaner GA, Boas FE, Petre EN, Yarmohammadi H, et al. (90)Y Resin Microspheres Radioembolization for Colon Cancer Liver Metastases Using Full-Strength Contrast Material. Cardiovasc Intervent Radiol. 2018;41(9):1419-27.
Shah JL, Zendejas-Ruiz IR, Thornton LM, Geller BS, Grajo JR, Collinsworth A, et al. Neoadjuvant transarterial radiation lobectomy for colorectal hepatic metastases: a small cohort analysis on safety, efficacy, and radiopathologic correlation. J Gastrointest Oncol. 2017;8(3):E43-e51.
Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4:198.
Shibata T, Yamamoto Y, Yamamoto N, Maetani Y, Shibata T, Ikai I, et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: incidence and risk factors. J Vasc Interv Radiol. 2003;14(12):1535-42.
Elias D, Di Pietroantonio D, Gachot B, Menegon P, Hakime A, De Baere T. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30(6-7):823-7.
Huang SY, Philip A, Richter MD, Gupta S, Lessne ML, Kim CY. Prevention and management of infectious complications of percutaneous interventions. Semin Intervent Radiol. 2015;32(2):78-88.
Kim W, Clark TW, Baum RA, Soulen MC. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(8):965-8.
Westcott MA, Coldwell DM, Liu DM, Zikria JF. The development, commercialization, and clinical context of yttrium-90 radiolabeled resin and glass microspheres. Advances in radiation oncology. 2016;1(4):351-64.
Allimant C, Kafrouni M, Delicque J, Ilonca D, Cassinotto C, Assenat E, et al. Tumor Targeting and Three-Dimensional Voxel-Based Dosimetry to Predict Tumor Response, Toxicity, and Survival after Yttrium-90 Resin Microsphere Radioembolization in Hepatocellular Carcinoma. J Vasc Interv Radiol. 2018;29(12):1662-70.e4.
Acknowledgements
This research was supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. E. Ziv: Research grants from SIR, RSNA, NANETS, Cycle for survival, MSK Society, MSK FGI, AACR, Ethicon, and Novartis. All conflicts of interest are outside the submitted work. Dr. C.T. Sofocleous: Research Support from National Institute of Health (NIH, R01 CA240569-01). Research support from industry: SIRTEX Medical Inc, BTG and Ethicon J&J. Dr. Sofocleous is consultant/ member of advisory board for: J&J/ Ethicon, Terumo, BTG/Boston Scientific, SIRTEX, Varian. All conflicts of interest are outside the submitted work. Dr. F.E. Boas is a co-founder of Claripacs, LLC. He received research funding (investigator-initiated) from Guerbet. He received research support (investigator-initiated) from GE. He received research supplies (investigator-initiated) from Bayer. He received a research grant and speaker fees from Society of Interventional Oncology, which were sponsored by Guerbet. He attended research meetings sponsored by Guerbet. He is an investor in Labdoor, Qventus, CloudMedx, Notable Labs, and Xgenomes. He is the inventor and assignee on US patent 8233586, and is an inventor on US provisional patent applications 62/754,139 and 62/817,116. All conflicts of interest are outside the submitted work. Other authors have co conflicts of interest.
Ethical approval
IRB approval was obtained for this retrospective cohort study.
Consent for publication
All authors provided their consent for publication.
Informed consent
For this type of study formal patient informed consent and consent for publication are not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurilova, I., Bendet, A., Fung, E.K. et al. Radiation segmentectomy of hepatic metastases with Y-90 glass microspheres. Abdom Radiol 46, 3428–3436 (2021). https://doi.org/10.1007/s00261-021-02956-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-02956-6