Abstract
Purpose
In the clinical management of patients with locally advanced rectal cancer (LARC), the early identification of poor and good responders after neoadjuvant chemoradiotherapy (N-CRT) is essential. Therefore, we developed and validated predictive models including MRI findings from the structured report template, clinical and radiomics parameters to differentiate between poor and good responders in patients with locally advanced rectal cancer who underwent neoadjuvant chemoradiotherapy.
Methods
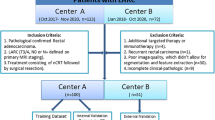
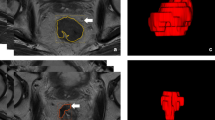
Preoperative multiparametric MRI from 183 patients with locally advanced rectal cancer (122 in the training cohort, 61 in the validation cohort) was included in this retrospective study. After preprocessing, radiomic features were extracted and two methods of feature selection was applied to reduce the number of radiomics features. Logistic regression (LR) and random forest (RF) machine learning classifiers were trained to identify good responders from poor responders. Multivariable logistic regression analysis was used to incorporate the radiomic signature and clinical risk factors into a nomogram. Classifier performance was evaluated using the area under the curve (AUC), accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Results
For the differentiation of poor and good responders, the radiomics model with an LR classifier achieved AUCs of 0.869 and 0.842 for the training and validation cohorts, respectively. The nomogram showed the highest reproducibility and prognostic ability in the training and validation cohorts, with AUCs of 0.923 (95% confidence interval, 0.872–0.975) and 0.898 (0.819–0.978), respectively. Additionally, the nomogram achieved significant risk stratification of patients in respect to progression free survival (PFS).
Conclusions
The nomogram accurately differentiated good and poor responders in patients with LARC undergoing N-CRT, and showed significant performance for predicting PFS.







Similar content being viewed by others
Abbreviations
- LARC:
-
Locally advanced rectal cancer
- TME:
-
Total mesorectal excision
- pCR:
-
Pathological complete response
- TRG:
-
Tumor response grading
- DWI:
-
Diffusion-weighted imaging
- CE-T1WI:
-
Contrast-enhanced T1-weighted imaging
- VOI:
-
Volume of interest
- mRMR:
-
Minimum redundancy maximum relevance feature selection
- LASSO:
-
Least absolute shrinkage and selection operator
- CEA:
-
Carcinoembryonic antigen
- CA199:
-
Carbohydrate antigen 199
- CRM:
-
Circumferential resection margin
- EMVI:
-
Extramural vascular invasion
- Rad-score:
-
Radiomics score
- PFS:
-
Progression free survival
- CI:
-
Confidence interval
- ICC:
-
Intraclass correlation coefficient
- TNM:
-
Tumor-node-metastasis
- LR:
-
Logistic regression
- RF:
-
Random forest
References
Mozafar M, Adhami F, Atqiaee K, Lotfollahzadeh S, Sobhiyeh M, Amraei R, et al. Neo-adjuvant chemoradiotherapy; an opportunity in sphincter preserving procedure for rectal cancer. Gastroenterology and hepatology from bed to bench 2014;7(1):32-7.
Ferrandina G, Palluzzi E, Gallotta V, Gambacorta M, Autorino R, Turco L, et al. Neo-adjuvant platinum-based chemotherapy followed by chemoradiation and radical surgery in locally advanced cervical cancer (Lacc) patients: A phase II study.. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2018;44(7):1062-8.
Chu K, Schoetz D. What impact might general surgery practice patterns of colon and rectal surgeons have on future training? Diseases of the colon and rectum 2007;50(8):1250-4.
Albert M, Monson J. Critical concepts and important anatomic landmarks encountered during transanal total mesorectal excision (taTME): toward the mastery of a new operation for rectal cancer surgery. Techniques in coloproctology 2016;20(7):483-94.
Koessler T, Puppa G, Fernandez E, Ho L, Dietrich P, Zilli T, et al. Early closure of fistula using neo-adjuvant intra-arterial chemotherapy in locally advanced anal cancer. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2017;49(11):1262-6.
Fu X, Yang J, Liu D, Li J, Zhang J, Huo Y, et al. Efficacy of Neo-Adjuvant Chemoradiotherapy for Resectable Pancreatic Adenocarcinoma: A PRISMA-Compliant Meta-Analysis and Systematic Review. Medicine 2016;95(15):e3009.
Kennelly R, Heeney A, White A, Fennelly D, Sheahan K, Hyland J, et al. A prospective analysis of patient outcome following treatment of T3 rectal cancer with neo-adjuvant chemoradiotherapy and transanal excision. International journal of colorectal disease 2012;27(6):759-64.
Ward WH, Sigurdson ER, Esposito AC, Ruth KJ, Manstein SM, Sorenson EC, et al. Pathologic response following treatment for locally advanced rectal cancer: Does location matter? J Surg Res 2018;224:215-21.
Couwenberg AM, Burbach JPM, van Grevenstein WMU, Smits AB, Consten ECJ, Schiphorst AHW, et al. Effect of Neoadjuvant Therapy and Rectal Surgery on Health-related Quality of Life in Patients With Rectal Cancer During the First 2 Years After Diagnosis. Clin Colorectal Cancer 2018;17(3):e499-e512.
Figueiredo N, Panteleimonitis S, Popeskou S, Cunha JF, Qureshi T, Beets GL, et al. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J Surg Oncol 2018;44(4):484-9.
Du D, Su Z, Wang D, Liu W, Wei Z. Optimal Interval to Surgery After Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Systematic Review and Meta-analysis. Clin Colorectal Cancer 2018;17(1):13-24.
Park YW, Choi YS, Ahn SS, Chang JH, Kim SH, Lee SK. Radiomics MRI Phenotyping with Machine Learning to Predict the Grade of Lower-Grade Gliomas: A Study Focused on Nonenhancing Tumors. Korean J Radiol 2019;20(9):1381-9.
Ge L, Chen Y, Yan C, Zhao P, Zhang P, A R, et al. Study Progress of Radiomics With Machine Learning for Precision Medicine in Bladder Cancer Management. Front Oncol 2019;9:1296.
Qi Y, Zhang S, Wei J, Zhang G, Lei J, Yan W, et al. Multiparametric MRI-Based Radiomics for Prostate Cancer Screening With PSA in 4-10 ng/mL to Reduce Unnecessary Biopsies. J Magn Reson Imaging 2019.
Mashayekhi R, Parekh VS, Faghih M, Singh VK, Jacobs MA, Zaheer A. Radiomic features of the pancreas on CT imaging accurately differentiate functional abdominal pain, recurrent acute pancreatitis, and chronic pancreatitis. Eur J Radiol 2019;123:108778.
Zhang P, Feng Z, Cai W, You H, Fan C, Lv W, et al. T2-Weighted Image-Based Radiomics Signature for Discriminating Between Seminomas and Nonseminoma. Front Oncol 2019;9:1330.
Cui Y, Yang X, Shi Z, Yang Z, Du X, Zhao Z, et al. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol 2019;29(3):1211-20.
Liu Z, Zhang XY, Shi YJ, Wang L, Zhu HT, Tang Z, et al. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin Cancer Res 2017;23(23):7253-62.
Li Y, Liu W, Pei Q, Zhao L, Gungor C, Zhu H, et al. Predicting pathological complete response by comparing MRI-based radiomics pre- and postneoadjuvant radiotherapy for locally advanced rectal cancer. Cancer Med 2019.
Mandard AM DF, Mandard JC, Marnay J, Henry-Amar M, Petiot JF. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer Imaging 1994;73:2680-6.
Tang X, Jiang W, Li H, Xie F, Dong A, Liu L, et al. Predicting poor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer: Model constructed using pre-treatment MRI features of structured report template. Radiotherapy and Oncology 2020;148:97-106.
Avanzo M, Stancanello J, El Naqa I. Beyond imaging: The promise of radiomics. Phys Med 2017;38:122-39.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14(12):749-62.
Arimura H, Soufi M, Kamezawa H, Ninomiya K, Yamada M. Radiomics with artificial intelligence for precision medicine in radiation therapy. J Radiat Res 2019;60(1):150-7.
Peng L, Parekh V, Huang P, Lin DD, Sheikh K, Baker B, et al. Distinguishing True Progression From Radionecrosis After Stereotactic Radiation Therapy for Brain Metastases With Machine Learning and Radiomics. Int J Radiat Oncol Biol Phys 2018;102(4):1236-43.
Park H, Lim Y, Ko ES, Cho HH, Lee JE, Han BK, et al. Radiomics Signature on Magnetic Resonance Imaging: Association with Disease-Free Survival in Patients with Invasive Breast Cancer. Clin Cancer Res 2018;24(19):4705-14.
Wilson R, Devaraj A. Radiomics of pulmonary nodules and lung cancer. Transl Lung Cancer Res 2017;6(1):86-91.
Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, et al. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology 2018;287(3):833-43.
Shi L, Zhang Y, Nie K, Sun X, Niu T, Yue N, et al. Machine learning for prediction of chemoradiation therapy response in rectal cancer using pre-treatment and mid-radiation multi-parametric MRI. Magn Reson Imaging 2019;61:33-40.
Fujii S, Nougaret S, Escal L, Azria D, Assenat E, Rouanet P, et al. MR imaging of locally advanced low rectal cancer: Relationships between imaging findings and the pathological tumor regression grade. J Magn Reson Imaging 2015;42(2):421-6.
Sato S, Kato T, Tanaka JI. Defining the distal margin of rectal cancer for surgical planning. J Gastrointest Oncol 2017;8(1):194-8.
Oberholzer K, Junginger T, Heintz A, Kreft A, Hansen T, Lollert A, et al. Rectal Cancer: MR imaging of the mesorectal fascia and effect of chemoradiation on assessment of tumor involvement. J Magn Reson Imaging 2012;36(3):658-63.
Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol 2012;19(7):2212-23.
Glynne-Jones R, Mawdsley S, Novell JR. The clinical significance of the circumferential resection margin following preoperative pelvic chemo-radiotherapy in rectal cancer: why we need a common language. Colorectal Dis 2006;8(9):800-7.
Depypere L, Moons J, Lerut T, De Hertogh G, Peters C, Sagaert X, et al. Prognostic value of the circumferential resection margin and its definitions in esophageal cancer patients after neoadjuvant chemoradiotherapy. Dis Esophagus 2018;31(2).
Wang S, Li XT, Zhang XY, Sun RJ, Qu YH, Zhu HC, et al. MRI evaluation of extramural vascular invasion by inexperienced radiologists. Br J Radiol 2019;92(1104):20181055.
Zhang XY, Wang S, Li XT, Wang YP, Shi YJ, Wang L, et al. MRI of Extramural Venous Invasion in Locally Advanced Rectal Cancer: Relationship to Tumor Recurrence and Overall Survival. Radiology 2018;289(3):677-85.
Chand M, Evans J, Swift RI, Tekkis PP, West NP, Stamp G, et al. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg 2015;261(3):473-9.
Lee ES, Kim MJ, Park SC, Hur BY, Hyun JH, Chang HJ, et al. Magnetic Resonance Imaging-Detected Extramural Venous Invasion in Rectal Cancer before and after Preoperative Chemoradiotherapy: Diagnostic Performance and Prognostic Significance. Eur Radiol 2018;28(2):496-505.
Schurink N, Lambregts D, Beets-Tan R. Diffusion-weighted imaging in rectal cancer: current applications and future perspectives. Br J Radiol 2018:20180655.
De Felice F, Magnante AL, Musio D, Ciolina M, De Cecco CN, Rengo M, et al. Diffusion-weighted magnetic resonance imaging in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Eur J Surg Oncol 2017;43(7):1324-9.
Peng Y, Li Z, Tang H, Wang Y, Hu X, Shen Y, et al. Comparison of reduced field-of-view diffusion-weighted imaging (DWI) and conventional DWI techniques in the assessment of rectal carcinoma at 3.0T: Image quality and histological T staging. J Magn Reson Imaging 2018;47(4):967-75.
Birlik B, Obuz F, Elibol FD, Celik AO, Sokmen S, Terzi C, et al. Diffusion-weighted MRI and MR- volumetry–in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Magn Reson Imaging 2015;33(2):201-12.
Cai G, Xu Y, Zhu J, Gu WL, Zhang S, Ma XJ, et al. Diffusion-weighted magnetic resonance imaging for predicting the response of rectal cancer to neoadjuvant concurrent chemoradiation. World J Gastroenterol 2013;19(33):5520-7.
Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29(28):3753-60.
Kong JC, Guerra GR, Warrier SK, Lynch AC, Michael M, Ngan SY, et al. Prognostic value of tumour regression grade in locally advanced rectal cancer: a systematic review and meta-analysis. Colorectal Dis 2018;20(7):574-85.
Fokas E, Ströbel P, Fietkau R, Ghadimi M, Liersch T, Grabenbauer GG, et al. Tumor Regression Grading After Preoperative Chemoradiotherapy as a Prognostic Factor and Individual-Level Surrogate for Disease-Free Survival in Rectal Cancer. JNCI: Journal of the National Cancer Institute 2017;109(12).
Huh JW, Kim HC, Kim SH, Park YA, Cho YB, Yun SH, et al. Tumor regression grade as a clinically useful outcome predictor in patients with rectal cancer after preoperative chemoradiotherapy. Surgery 2019;165(3):579-85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in connection with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Liu, X., Hu, B. et al. Development and validation of an MRI-based radiomic nomogram to distinguish between good and poor responders in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. Abdom Radiol 46, 1805–1815 (2021). https://doi.org/10.1007/s00261-020-02846-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02846-3