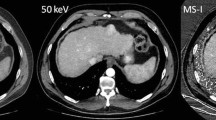
Abstract
Dual-energy CT (DECT) can be defined as the use of two different energy levels to identify and quantify material composition. Since its inception, DECT has benefited from remarkable improvements in hardware and clinical applications. DECT enables accurate identification and quantification of multiple materials, including fat, iron, and iodine. As a consequence, multiple studies have investigated the potential role of DECT in the assessment of diffuse liver diseases. While this role is evolving, this article aims to review the most relevant literature on use of DECT for assessment of diffuse liver diseases. Moreover, the basic concepts on DECT techniques, types of image reconstruction, and DECT-dedicated software will be described, focusing on the areas that are most relevant for the evaluation of diffuse liver diseases. Also, we will review the evidence of added value of DECT in detection and assessment of hepatocellular carcinoma which is a known risk in patients with diffuse liver disease.






Similar content being viewed by others
References
Marcellin P, Kutala BK (2018) Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver International 38:2–6.
Kose S, Ersan G, Tatar B, et al (2015) Evaluation of percutaneous liver biopsy complications in patients with chronic viral hepatitis. The Eurasian Journal of Medicine 47:161.
Ratziu V, Charlotte F, Heurtier A, et al. (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128:1898–1906
Regev A, Berho M, Jeffers LJ, et al (2002) Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterology 97:2614–2618.
Yoon JH, Lee JM, Joo I, et al (2014) Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology 273:772–782.
De Cecco CN, Boll DT, Bolus DN, et al (2017) White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 4: Abdominal and Pelvic Applications. Journal of Computer Assisted Tomography 41:8–14.
Rutherford RA, Pullan BR, Isherwood I (1976) Measurement of effective atomic number and electron density using an EMI scanner. Neuroradiology 11:15–21.
Kruger RA, Riederer SJ, Mistretta CA (1977) Relative properties of tomography, K-edge imaging, and K-edge tomography. Medical Physics 4:244–249.
McCollough CH, Leng S, Yu L, Fletcher JG (2015) Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 276:637–653. https://doi.org/10.1148/radiol.2015142631
Patino M, Prochowski A, Agrawal MD, et al (2016) Material separation using dual-energy CT: current and emerging applications. RadioGraphics 36:1087–1105.
Sellerer T, Noël PB, Patino M, et al (2018) Dual-energy CT: a phantom comparison of different platforms for abdominal imaging. Eur Radiol 28:2745–2755.
Primak AN, Giraldo JCR, Eusemann CD, et al (2010) Dual-source dual-energy CT with additional tin filtration: dose and image quality evaluation in phantoms and in vivo. American Journal of Roentgenology 195:1164–1174.
Silva AC, Morse BG, Hara AK, et al (2011) Dual-Energy (Spectral) CT: Applications in Abdominal Imaging. RadioGraphics 31:1031–1046. https://doi.org/10.1148/rg.314105159
Wu L-M, Xu JR, Yin Y, Qu X-H (2010) Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World Journal of Gastroenterology: WJG 16:3957.
Grasruck M, Kappler S, Reinwand M, Stierstorfer K (2009) Dual energy with dual source CT and kVp switching with single source CT: a comparison of dual energy performance. International Society for Optics and Photonics, p 72583R
Wu E-H, Kim SY, Wang ZJ, et al (2016) Appearance and frequency of gas interface artifacts involving small bowel on rapid-voltage-switching dual-energy CT iodine-density images. American Journal of Roentgenology 206:301–306.
Fornaro J, Leschka S, Hibbeln D, et al (2011) Dual-and multi-energy CT: approach to functional imaging. Insights Imaging 2:149–159.
Hartman R, Kawashima A, Takahashi N, et al (2012) Applications of dual-energy CT in urologic imaging: an update. Radiologic Clinics of NA 50:191–205.
Goceri E, Shah ZK, Layman R, et al (2016) Quantification of liver fat: a comprehensive review. Computers in Biology and Medicine 71:174–189.
Loomba R, Sanyal AJ (2013) The global NAFLD epidemic. Nature Reviews Gastroenterology and Hepatology 10:686–690.
Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM (2007) Steatosis as a risk factor in liver surgery. Annals of Surgery 245:20.
Xu M-M, Brown RS (2015) Liver transplantation for the referring physician. Clinics in Liver Disease 19:135–153.
Brunt EM, Janney CG, Di Bisceglie AM, et al (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterology 94:2467–2474.
van Werven JR, Marsman HA, Nederveen AJ, et al (2010) Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 256:159–168.
Wang B, Gao Z, Zou Q, Li L (2003) Quantitative Diagnosis of Fatty Liver With Dual-Energy CT: An experimental study in rabbits. Acta Radiol 44:92–97.
Artz NS, Hines CD, Brunner ST, et al (2012) Quantification of hepatic steatosis with dual-energy computed tomography: comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob mouse. Invest Radiol 47:603.
Kramer H, Pickhardt PJ, Kliewer MA, et al (2017) Accuracy of liver fat quantification with advanced CT, MRI, and ultrasound techniques: prospective comparison with MR spectroscopy. American Journal of Roentgenology 208:92–100.
Patel BN, Kumbla RA, Berland LL, et al (2013) Material density hepatic steatosis quantification on intravenous contrast-enhanced rapid kilovolt (peak)–switching single-source dual-energy computed tomography. Journal of Computer Assisted Tomography 37:904–910.
Hyodo T, Yada N, Hori M, et al (2017) Multimaterial decomposition algorithm for the quantification of liver fat content by using fast-kilovolt-peak switching dual-energy CT: clinical evaluation. Radiology 283:108–118.
Hur BY, Lee JM, Hyunsik W, et al (2014) Quantification of the fat fraction in the liver using dual-energy computed tomography and multimaterial decomposition. Journal of Computer Assisted Tomography 38:845–852.
Guo Z, Blake GM, Li K, et al (2020) Liver Fat Content Measurement with Quantitative CT Validated against MRI Proton Density Fat Fraction: A Prospective Study of 400 Healthy Volunteers. Radiology 294:89–97.
Zheng X, Ren Y, Phillips WT, et al (2013) Assessment of hepatic fatty infiltration using spectral computed tomography imaging: a pilot study. Journal of Computer Assisted Tomography 37:134–141.
Pietrangelo A (2010) Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology 139:393–408.
Porter JB, de Witte T, Cappellini MD, Gattermann N (2016) New insights into transfusion-related iron toxicity: Implications for the oncologist. Critical Reviews in Oncology/Hematology 99:261–271.
Kew MC (2014) Hepatic iron overload and hepatocellular carcinoma. Liver Cancer 3:31–40.
Shander A, Sazama K (2010) Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion 50:1144–1155.
Henninger B, Alustiza J, Garbowski M, Gandon Y (2020) Practical guide to quantification of hepatic iron with MRI. Eur Radiol 30:383–393.
Werner S, Krauss B, Haberland U, et al (2019) Dual-energy CT for liver iron quantification in patients with haematological disorders. Eur Radiol 29:2868–2877.
Fischer MA, Gnannt R, Raptis D, et al (2011) Quantification of liver fat in the presence of iron and iodine: an ex vivo dual-energy CT study. Invest Radiol 46:351–358.
Joe E, Kim SH, Lee KB, et al (2012) Feasibility and accuracy of dual-source dual-energy CT for noninvasive determination of hepatic iron accumulation. Radiology 262:126–135.
Ma Q, Hu J, Yang W, Hou Y (2020) Dual-layer detector spectral CT versus magnetic resonance imaging for the assessment of iron overload in myelodysplastic syndromes and aplastic anemia. Jpn J Radiol 1–8.
Luo XF, Xie XQ, Cheng S, et al (2015) Dual-Energy CT for Patients Suspected of Having Liver Iron Overload: Can Virtual Iron Content Imaging Accurately Quantify Liver Iron Content? Radiology 277:95–103. https://doi.org/10.1148/radiol.2015141856
Luo XF, Yang Y, Yan J, et al (2015) Virtual iron concentration imaging based on dual-energy CT for noninvasive quantification and grading of liver iron content: An iron overload rabbit model study. Eur Radiol 25:2657–2664.
Abadia AF, Grant KL, Carey KE, et al (2017) Spatial Distribution of Iron Within the Normal Human Liver Using Dual-Source Dual-Energy CT Imaging. Invest Radiol 52:693–700.
Scaglione S, Kliethermes S, Cao G, et al (2015) The epidemiology of cirrhosis in the United States. Journal of Clinical Gastroenterology 49:690–696.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS (2019) Burden of liver diseases in the world. Journal of Hepatology 70:151–171.
Younossi ZM, Stepanova M, Younossi Y, et al (2020) Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 69:564–568.
Schuppan D, Afdhal NH (2008) Liver cirrhosis. The Lancet 371:838–851.
Horowitz JM, Venkatesh SK, Ehman RL, et al (2017) Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol 42:2037–2053.
Guo SL, Su LN, Zhai YN, et al (2017) The clinical value of hepatic extracellular volume fraction using routine multiphasic contrast-enhanced liver CT for staging liver fibrosis. Clinical Radiology 72:242–246.
Sofue K, Tsurusaki M, Mileto A, et al (2018) Dual-energy computed tomography for non-invasive staging of liver fibrosis: Accuracy of iodine density measurements from contrast-enhanced data. Hepatology Research 48:1008–1019.
Zissen MH, Wang ZJ, Yee J, et al (2013) Contrast-enhanced CT quantification of the hepatic fractional extracellular space: correlation with diffuse liver disease severity. American Journal of Roentgenology 201:1204–1210.
Varenika V, Fu Y, Maher JJ, et al (2013) Hepatic fibrosis: evaluation with semiquantitative contrast-enhanced CT. Radiology 266:151–158.
Bandula S, Punwani S, Rosenberg WM, et al (2015) Equilibrium contrast-enhanced CT imaging to evaluate hepatic fibrosis: initial validation by comparison with histopathologic sampling. Radiology 275:136–143.
Yoon JH, Lee JM, Klotz E, et al (2015) Estimation of hepatic extracellular volume fraction using multiphasic liver computed tomography for hepatic fibrosis grading. Invest Radiol 50:290–296.
Sahin S, Rowland M (2000) Estimation of aqueous distributional spaces in the dual perfused rat liver. The Journal of Physiology 528:199–207.
Kaza RK, Caoili EM, Cohan RH, Platt JF (2011) Distinguishing enhancing from nonenhancing renal lesions with fast kilovoltage-switching dual-energy CT. American Journal of Roentgenology 197:1375–1381.
Bottari A, Silipigni S, Carerj ML, et al (2020) Dual-source dual-energy CT in the evaluation of hepatic fractional extracellular space in cirrhosis. La radiologia medica 125:7–14.
Dong J, He F, Wang L, et al (2019) Iodine density changes in hepatic and splenic parenchyma in liver cirrhosis with dual energy CT (DECT): a preliminary study. Academic Radiology 26:872–877.
Shang S, Cao Q, Han X, et al (2020) Assessing Liver Hemodynamics in Children With Cholestatic Cirrhosis by Use of Dual-Energy Spectral CT. American Journal of Roentgenology 214:665–670.
Bak S, Kim JE, Bae K, et al Quantification of liver extracellular volume using dual-energy CT: utility for prediction of liver-related events in cirrhosis. Eur Radiol 1–10.
Chernyak V, Fowler KJ, Kamaya A, et al (2018) Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 289:816–830.
Purysko AS, Primak AN, Baker ME, et al (2014) Comparison of radiation dose and image quality from single-energy and dual-energy CT examinations in the same patients screened for hepatocellular carcinoma. Clinical Radiology 69:e538–e544.
Hanson GJ, Michalak GJ, Childs R, et al (2018) Low kV versus dual-energy virtual monoenergetic CT imaging for proven liver lesions: what are the advantages and trade-offs in conspicuity and image quality? A pilot study. Abdom Radiol 43:1404–1412.
Husarik DB, Gordic S, Desbiolles L, et al (2015) Advanced virtual monoenergetic computed tomography of hyperattenuating and hypoattenuating liver lesions: ex vivo and patient experience in various body sizes. Invest Radiol 50:695–702.
Shuman WP, Green DE, Busey JM, et al (2014) Dual-energy liver CT: effect of monochromatic imaging on lesion detection, conspicuity, and contrast-to-noise ratio of hypervascular lesions on late arterial phase. American Journal of Roentgenology 203:601–606.
De Cecco CN, Caruso D, Schoepf UJ, et al (2018) A noise-optimized virtual monoenergetic reconstruction algorithm improves the diagnostic accuracy of late hepatic arterial phase dual-energy CT for the detection of hypervascular liver lesions. Eur Radiol 28:3393–3404.
Yoon JH, Chang W, Lee ES, et al (2020) Double Low-Dose Dual-Energy Liver CT in Patients at High-Risk of HCC: A Prospective, Randomized, Single-Center Study. Invest Radiol 55:340–348.
Mileto A, Nelson RC, Samei E, et al (2014) Dual-energy MDCT in hypervascular liver tumors: effect of body size on selection of the optimal monochromatic energy level. American Journal of Roentgenology 203:1257–1264.
Matsuda M, Tsuda T, Kido T, et al (2018) Dual-energy computed tomography in patients with small hepatocellular carcinoma: utility of noise-reduced monoenergetic images for the evaluation of washout and image quality in the equilibrium phase. Journal of Computer Assisted Tomography 42:937–943.
Caruso D, De Cecco CN, Schoepf UJ, et al (2017) Can dual-energy computed tomography improve visualization of hypoenhancing liver lesions in portal venous phase? Assessment of advanced image-based virtual monoenergetic images. Journal of Clinical Imaging 41:118–124.
Lenga L, Lange M, Arendt CT, et al (2020) Can Dual-energy CT-based Virtual Monoenergetic Imaging Improve the Assessment of Hypodense Liver Metastases in Patients With Hepatic Steatosis? Academic Radiology
Pfeiffer D, Parakh A, Patino M, et al (2018) Iodine material density images in dual-energy CT: quantification of contrast uptake and washout in HCC. Abdom Radiol 43:3317–3323.
Lee J-A, Jeong WK, Kim Y, et al (2013) Dual-energy CT to detect recurrent HCC after TACE: initial experience of color-coded iodine CT imaging. European Journal of Radiology 82:569–576.
Lee SH, Lee JM, Kim KW, et al (2011) Dual-energy computed tomography to assess tumor response to hepatic radiofrequency ablation: potential diagnostic value of virtual noncontrast images and iodine maps. Invest Radiol 46:77–84.
Altenbernd J, Heusner TA, Ringelstein A, et al (2011) Dual-energy-CT of hypervascular liver lesions in patients with HCC: investigation of image quality and sensitivity. Eur Radiol 21:738–743.
Dai X, Schlemmer H-P, Schmidt B, et al (2013) Quantitative therapy response assessment by volumetric iodine-uptake measurement: initial experience in patients with advanced hepatocellular carcinoma treated with sorafenib. European Journal of Radiology 82:327–334.
Ascenti G, Sofia C, Mazziotti S, et al (2016) Dual-energy CT with iodine quantification in distinguishing between bland and neoplastic portal vein thrombosis in patients with hepatocellular carcinoma. Clinical Radiology 71:938-e1.
Qian LJ, Zhu J, Zhuang ZG, et al (2012) Differentiation of neoplastic from bland macroscopic portal vein thrombi using dual-energy spectral CT imaging: a pilot study. Eur Radiol 22:2178–2185.
Guimaraes LS, Fletcher JG, Harmsen WS, et al (2010) Appropriate patient selection at abdominal dual-energy CT using 80 kV: relationship between patient size, image noise, and image quality. Radiology 257:732–742.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.Y.E., A.M., B.M. and L.S.G. have nothing to disclose. P.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received Institutional Research Grant from Canon Medical Systems. There was no commercial funding for this study. All authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elbanna, K.Y., Mansoori, B., Mileto, A. et al. Dual-energy CT in diffuse liver disease: is there a role?. Abdom Radiol 45, 3413–3424 (2020). https://doi.org/10.1007/s00261-020-02702-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02702-4